The structure and function of cardiac t-tubules in health and disease
- PMID: 21697171
- PMCID: PMC3145195
- DOI: 10.1098/rspb.2011.0624
The structure and function of cardiac t-tubules in health and disease
Abstract
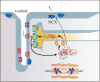
The transverse tubules (t-tubules) are invaginations of the cell membrane rich in several ion channels and other proteins devoted to the critical task of excitation-contraction coupling in cardiac muscle cells (cardiomyocytes). They are thought to promote the synchronous activation of the whole depth of the cell despite the fact that the signal to contract is relayed across the external membrane. However, recent work has shown that t-tubule structure and function are complex and tightly regulated in healthy cardiomyocytes. In this review, we outline the rapidly accumulating knowledge of its novel roles and discuss the emerging evidence of t-tubule dysfunction in cardiac disease, especially heart failure. Controversy surrounds the t-tubules' regulatory elements, and we draw attention to work that is defining these elements from the genetic and the physiological levels. More generally, this field illustrates the challenges in the dissection of the complex relationship between cellular structure and function.
This journal is © 2011 The Royal Society
Figures





References
-
- Soeller C., Cannell M. B. 1999. Examination of the transverse tubular system in living cardiac rat myocytes by 2-photon microscopy and digital image-processing techniques. Circ. Res. 1984, 266–275 - PubMed
-
- Orchard C. H., Pasek M., Brette F. 2009. The role of mammalian cardiac t-tubules in excitation–contraction coupling: experimental and computational approaches. Exp. Physiol. 94, 509–51910.1113/expphysiol.2008.043984 (doi:10.1113/expphysiol.2008.043984) - DOI - DOI - PubMed
-
- Linder E. 1956. Die submikroskopische Morphologie des Herzmuskels. Zeitschrift Zellforsch 45, 702–746 - PubMed
-
- Sperelakis N., Rubio R. 1971. An orderly lattice of axial tubules which interconnect adjacent transverse tubules in guinea-pig ventricular myocardium. J. Mol. Cell. Cardiol. 2, 211–22010.1016/0022-2828(71)90054-X (doi:10.1016/0022-2828(71)90054-X) - DOI - DOI - PubMed
-
- Porter K., Palade G. 1957. Studies on the endoplasmic reticulum. III. Its form and distribution in striated muscle cells. J. Biophys. Biochem. Cytol. 3, 269–30010.1083/jcb.3.2.269 (doi:10.1083/jcb.3.2.269) - DOI - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

