Multisite comparison of high-sensitivity multiplex cytokine assays
- PMID: 21697338
- PMCID: PMC3147360
- DOI: 10.1128/CVI.05032-11
Multisite comparison of high-sensitivity multiplex cytokine assays
Abstract
The concentrations of cytokines in human serum and plasma can provide valuable information about in vivo immune status, but low concentrations often require high-sensitivity assays to permit detection. The recent development of multiplex assays, which can measure multiple cytokines in one small sample, holds great promise, especially for studies in which limited volumes of stored serum or plasma are available. Four high-sensitivity cytokine multiplex assays on a Luminex (Bio-Rad, BioSource, Linco) or electrochemiluminescence (Meso Scale Discovery) platform were evaluated for their ability to detect circulating concentrations of 13 cytokines, as well as for laboratory and lot variability. Assays were performed in six different laboratories utilizing archived serum from HIV-uninfected and -infected subjects from the Multicenter AIDS Cohort Study (MACS) and the Women's Interagency HIV Study (WIHS) and commercial plasma samples spanning initial HIV viremia. In a majority of serum samples, interleukin-6 (IL-6), IL-8, IL-10, and tumor necrosis factor alpha were detectable with at least three kits, while IL-1β was clearly detected with only one kit. No single multiplex panel detected all cytokines, and there were highly significant differences (P < 0.001) between laboratories and/or lots with all kits. Nevertheless, the kits generally detected similar patterns of cytokine perturbation during primary HIV viremia. This multisite comparison suggests that current multiplex assays vary in their ability to measure serum and/or plasma concentrations of cytokines and may not be sufficiently reproducible for repeated determinations over a long-term study or in multiple laboratories but may be useful for longitudinal studies in which relative, rather than absolute, changes in cytokines are important.
Figures

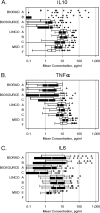


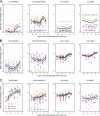
References
-
- Breen E. C., et al. 2006. Elevated serum soluble CD30 (sCD30) precedes the development of AIDS-associated non-Hodgkin's B cell lymphoma. Tumor Biol. 27:187–194 - PubMed
-
- Chowdhury F., Williams A., Johnson P. 2009. Validation and comparison of two multiplex technologies, Luminex and Mesoscale Discovery, for human cytokine profiling. J. Immunol. Methods 340:55–64 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 AI035042/AI/NIAID NIH HHS/United States
- U01 AI067854/AI/NIAID NIH HHS/United States
- U01 AI035004/AI/NIAID NIH HHS/United States
- U01-HD-32632/HD/NICHD NIH HHS/United States
- U01-AI-35041/AI/NIAID NIH HHS/United States
- U01 AI035041/AI/NIAID NIH HHS/United States
- U01-AI-35004/AI/NIAID NIH HHS/United States
- U01-AI-31834/AI/NIAID NIH HHS/United States
- P30 AG028748/AG/NIA NIH HHS/United States
- P30 AI027763/AI/NIAID NIH HHS/United States
- U01-AI-35039/AI/NIAID NIH HHS/United States
- U01 AI035040/AI/NIAID NIH HHS/United States
- U01 AI034993/AI/NIAID NIH HHS/United States
- U01 AI035039/AI/NIAID NIH HHS/United States
- U01-AI-35043/AI/NIAID NIH HHS/United States
- P30-AG028748/AG/NIA NIH HHS/United States
- U01-AI-35042/AI/NIAID NIH HHS/United States
- U01-AI-42590/AI/NIAID NIH HHS/United States
- UL1 RR025005/RR/NCRR NIH HHS/United States
- U01 AI031834/AI/NIAID NIH HHS/United States
- UL1 RR024131/RR/NCRR NIH HHS/United States
- U01 AI034989/AI/NIAID NIH HHS/United States
- U01 AI034994/AI/NIAID NIH HHS/United States
- U19 AI067854/AI/NIAID NIH HHS/United States
- UL1-RR025005/RR/NCRR NIH HHS/United States
- U01-AI-35040/AI/NIAID NIH HHS/United States
- U01 AI035043/AI/NIAID NIH HHS/United States
- U01-AI-34993/AI/NIAID NIH HHS/United States
- P30-AI027763/AI/NIAID NIH HHS/United States
- U01-AI-34989/AI/NIAID NIH HHS/United States
- U01 HD032632/HD/NICHD NIH HHS/United States
- U01 AI042590/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

