Differential control of presynaptic CaMKII activation and translocation to active zones
- PMID: 21697360
- PMCID: PMC3123710
- DOI: 10.1523/JNEUROSCI.0550-11.2011
Differential control of presynaptic CaMKII activation and translocation to active zones
Abstract
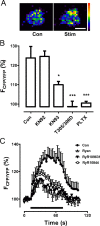
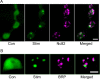
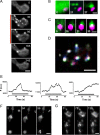
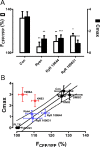
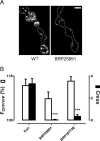
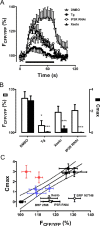
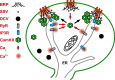
The release of neurotransmitters, neurotrophins, and neuropeptides is modulated by Ca(2+) mobilization from the endoplasmic reticulum (ER) and activation of Ca(2+)/calmodulin-dependent protein kinase II (CaMKII). Furthermore, when neuronal cultures are subjected to prolonged depolarization, presynaptic CaMKII redistributes from the cytoplasm to accumulate near active zones (AZs), a process that is reminiscent of CaMKII translocation to the postsynaptic side of the synapse. However, it is not known how presynaptic CaMKII activation and translocation depend on neuronal activity and ER Ca(2+) release. Here these issues are addressed in Drosophila motoneuron terminals by imaging a fluorescent reporter of CaMKII activity and subcellular distribution. We report that neuronal excitation acts with ER Ca(2+) stores to induce CaMKII activation and translocation to a subset of AZs. Surprisingly, activation is slow, reflecting T286 autophosphorylation and the function of presynaptic ER ryanodine receptors (RyRs) and inositol trisphosphate receptors (IP3Rs). Furthermore, translocation is not simply proportional to CaMKII activity, as T286 autophosphorylation promotes activation, but does not affect translocation. In contrast, RNA interference-induced knockdown of the AZ scaffold protein Bruchpilot disrupts CaMKII translocation without affecting activation. Finally, RyRs comparably stimulate both activation and translocation, but IP3Rs preferentially promote translocation. Thus, Ca(2+) provided by different presynaptic ER Ca(2+) release channels is not equivalent. These results suggest that presynaptic CaMKII activation depends on autophosphorylation and global Ca(2+) in the terminal, while translocation to AZs requires Ca(2+) microdomains generated by IP3Rs.
Figures

Similar articles
-
Presynaptic ryanodine receptor-CamKII signaling is required for activity-dependent capture of transiting vesicles.J Mol Neurosci. 2009 Feb;37(2):146-50. doi: 10.1007/s12031-008-9080-8. Epub 2008 Jul 1. J Mol Neurosci. 2009. PMID: 18592416 Free PMC article.
-
Changes in the distribution of calcium calmodulin-dependent protein kinase II at the presynaptic bouton after depolarization.Brain Cell Biol. 2006 Jun;35(2-3):117-24. doi: 10.1007/s11068-007-9012-5. Epub 2007 Sep 20. Brain Cell Biol. 2006. PMID: 17957478
-
Intracellular Ca(2+) and Ca(2+)/calmodulin-dependent kinase II mediate acute potentiation of neurotransmitter release by neurotrophin-3.J Cell Biol. 2000 May 15;149(4):783-92. doi: 10.1083/jcb.149.4.783. J Cell Biol. 2000. PMID: 10811820 Free PMC article.
-
Regulation of synaptic transmission by presynaptic CaMKII and BK channels.Mol Neurobiol. 2008 Oct;38(2):153-66. doi: 10.1007/s12035-008-8039-7. Epub 2008 Aug 29. Mol Neurobiol. 2008. PMID: 18759010 Free PMC article. Review.
-
Presence and functional significance of presynaptic ryanodine receptors.Prog Neurobiol. 2003 Apr;69(6):391-418. doi: 10.1016/s0301-0082(03)00053-4. Prog Neurobiol. 2003. PMID: 12880633 Review.
Cited by
-
The CaMKII/NMDAR complex as a molecular memory.Mol Brain. 2013 Feb 14;6:10. doi: 10.1186/1756-6606-6-10. Mol Brain. 2013. PMID: 23410178 Free PMC article. Review.
-
Orai-mediated calcium entry determines activity of central dopaminergic neurons by regulation of gene expression.Elife. 2024 Jan 30;12:RP88808. doi: 10.7554/eLife.88808. Elife. 2024. PMID: 38289659 Free PMC article.
-
Recombinant probes reveal dynamic localization of CaMKIIα within somata of cortical neurons.J Neurosci. 2013 Sep 4;33(36):14579-90. doi: 10.1523/JNEUROSCI.2108-13.2013. J Neurosci. 2013. PMID: 24005308 Free PMC article.
-
A computational model of motor neuron degeneration.Neuron. 2014 Aug 20;83(4):975-88. doi: 10.1016/j.neuron.2014.07.001. Epub 2014 Jul 31. Neuron. 2014. PMID: 25088365 Free PMC article.
-
Intracellular amyloid β oligomers impair organelle transport and induce dendritic spine loss in primary neurons.Acta Neuropathol Commun. 2015 Aug 21;3:51. doi: 10.1186/s40478-015-0230-2. Acta Neuropathol Commun. 2015. PMID: 26293809 Free PMC article.
References
-
- Blaustein MP, Golovina VA. Structural complexity and functional diversity of endoplasmic reticulum Ca2+ stores. Trends Neurosci. 2001;24:602–608. - PubMed
-
- Bradshaw JM, Hudmon A, Schulman H. Chemical quenched flow kinetic studies indicate an intraholoenzyme autophosphorylation mechanism for Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 2002;277:20991–20998. - PubMed
-
- Colbran RJ, Soderling TR. Calcium/calmodulin-independent autophosphorylation sites of calcium/calmodulin-dependent protein kinase II. Studies on the effect of phosphorylation of threonine 305/306 and serine 314 on calmodulin binding using synthetic peptides. J Biol Chem. 1990;265:11213–11219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
