Role of the CaMKII/NMDA receptor complex in the maintenance of synaptic strength
- PMID: 21697368
- PMCID: PMC3138556
- DOI: 10.1523/JNEUROSCI.1250-11.2011
Role of the CaMKII/NMDA receptor complex in the maintenance of synaptic strength
Abstract
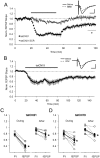
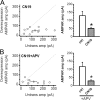
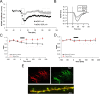
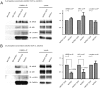
During long-term potentiation (LTP), synapses undergo stable changes in synaptic strength. The molecular memory processes that maintain strength have not been identified. One hypothesis is that the complex formed by the Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) and the NMDA-type glutamate receptor (NMDAR) is a molecular memory at the synapse. To establish a molecule as a molecular memory, it must be shown that interfering with the molecule produces a persistent reversal of LTP. We used the CN class of peptides that inhibit CaMKII binding to the NR2B subunit in vitro to test this prediction in rat hippocampal slices. We found that CN peptides can reverse saturated LTP, allowing additional LTP to be induced. The peptide also produced a persistent reduction in basal transmission. We then tested whether CN compounds actually affect CaMKII binding in living cells. Application of CN peptide to slice cultures reduced the amount of CaMKII concentrated in spines, consistent with delocalization of the kinase from a binding partner in the spine. To more specifically assay the binding of CaMKII to the NMDAR, we used coimmunoprecipitation methods. We found that CN peptide decreased synaptic strength only at concentrations necessary to disrupt the CaMKII/NMDAR complex, but not at lower concentrations sufficient to inhibit CaMKII activity. Importantly, both the reduction of the complex and the reduction of synaptic strength persisted after removal of the inhibitor. These results support the hypothesis that the CaMKII/NMDAR complex has switch-like properties that are important in the maintenance of synaptic strength.
Figures


References
-
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. - PubMed
-
- Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature. 2001;411:801–805. - PubMed
-
- Carlier E, Dargent B, De Waard M, Couraud F. Na(+) channel regulation by calmodulin kinase II in rat cerebellar granule cells. Biochem Biophys Res Commun. 2000;274:394–399. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
