Cortical glial fibrillary acidic protein-positive cells generate neurons after perinatal hypoxic injury
- PMID: 21697371
- PMCID: PMC3142780
- DOI: 10.1523/JNEUROSCI.0518-11.2011
Cortical glial fibrillary acidic protein-positive cells generate neurons after perinatal hypoxic injury
Abstract
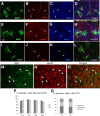
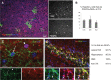
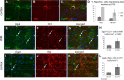
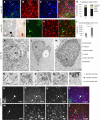
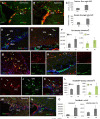
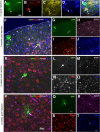
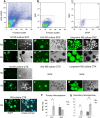
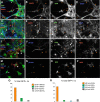
Glial fibrillary acidic protein-positive (GFAP(+)) cells give rise to new neurons in the neurogenic niches; whether they are able to generate neurons in the cortical parenchyma is not known. Here, we use genetic fate mapping to examine the progeny of GFAP(+) cells after postnatal hypoxia, a model for the brain injury observed in premature children. After hypoxia, immature cortical astroglia underwent a shift toward neuronal fate and generated cortical excitatory neurons that appeared synaptically integrated into the circuitry. Fate-mapped cortical GFAP(+) cells derived ex vivo from hypoxic, but not normoxic, mice were able to form pluripotent, long-term self-renewing neurospheres. Similarly, exposure to low oxygen conditions in vitro induced stem-cell-like potential in immature cortical GFAP(+) cells. Our data support the conclusion that hypoxia promotes pluripotency in GFAP(+) cells in the cortical parenchyma. Such plasticity possibly explains the cognitive recovery found in some preterm children.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
