Controlling specific locomotor behaviors through multidimensional monoaminergic modulation of spinal circuitries
- PMID: 21697376
- PMCID: PMC3422212
- DOI: 10.1523/JNEUROSCI.5796-10.2011
Controlling specific locomotor behaviors through multidimensional monoaminergic modulation of spinal circuitries
Abstract
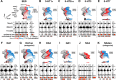
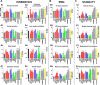
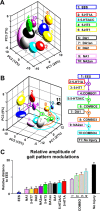
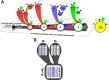
Descending monoaminergic inputs markedly influence spinal locomotor circuits, but the functional relationships between specific receptors and the control of walking behavior remain poorly understood. To identify these interactions, we manipulated serotonergic, dopaminergic, and noradrenergic neural pathways pharmacologically during locomotion enabled by electrical spinal cord stimulation in adult spinal rats in vivo. Using advanced neurobiomechanical recordings and multidimensional statistical procedures, we reveal that each monoaminergic receptor modulates a broad but distinct spectrum of kinematic, kinetic, and EMG characteristics, which we expressed into receptor-specific functional maps. We then exploited this catalog of monoaminergic tuning functions to devise optimal pharmacological combinations to encourage locomotion in paralyzed rats. We found that, in most cases, receptor-specific modulatory influences summed near algebraically when stimulating multiple pathways concurrently. Capitalizing on these predictive interactions, we elaborated a multidimensional monoaminergic intervention that restored coordinated hindlimb locomotion with normal levels of weight bearing and partial equilibrium maintenance in spinal rats. These findings provide new perspectives on the functions of and interactions between spinal monoaminergic receptor systems in producing stepping, and define a framework to tailor pharmacotherapies for improving neurological functions after CNS disorders.
Figures




References
-
- Agnati LF, Fuxe K. Volume transmission as a key feature of information handling in the central nervous system possible new interpretative value of the Turing's B-type machine. Prog Brain Res. 2000;125:3–19. - PubMed
-
- Agnati LF, Guidolin D, Guescini M, Genedani S, Fuxe K. Understanding wiring and volume transmission. Brain Res Rev. 2010;64:137–159. - PubMed
-
- Antri M, Mouffle C, Orsal D, Barthe JY. 5-HT1A receptors are involved in short- and long-term processes responsible for 5-HT-induced locomotor function recovery in chronic spinal rat. Eur J Neurosci. 2003;18:1963–1972. - PubMed
-
- Barbeau H, Rossignol S. Initiation and modulation of the locomotor pattern in the adult chronic spinal cat by noradrenergic, serotonergic and dopaminergic drugs. Brain Res. 1991;546:250–260. - PubMed
-
- Carhart MR, He J, Herman R, D'Luzansky S, Willis WT. Epidural spinal-cord stimulation facilitates recovery of functional walking following incomplete spinal-cord injury. IEEE Trans Neural Syst Rehabil Eng. 2004;12:32–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
