Spontaneous age-related neurite branching in Caenorhabditis elegans
- PMID: 21697377
- PMCID: PMC3148144
- DOI: 10.1523/JNEUROSCI.6606-10.2011
Spontaneous age-related neurite branching in Caenorhabditis elegans
Abstract
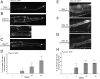
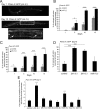
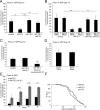
The analysis of morphological changes that occur in the nervous system during normal aging could provide insight into cognitive decline and neurodegenerative disease. Previous studies have suggested that the nervous system of Caenorhabditis elegans maintains its structural integrity with age despite the deterioration of surrounding tissues. Unexpectedly, we observed that neurons in aging animals frequently displayed ectopic branches and that the prevalence of these branches increased with time. Within age-matched populations, the branching of mechanosensory neurons correlated with decreased response to light touch and decreased mobility. The incidence of branching was influenced by two pathways that can affect the rate of aging, the Jun kinase pathway and the insulin/IGF-1 pathway. Loss of Jun kinase signaling, which slightly shortens lifespan, dramatically increased and accelerated the frequency of neurite branching. Conversely, inhibition of the daf-2 insulin/IGF-1-like signaling pathway, which extends lifespan, delayed and suppressed branching, and this delay required DAF-16/FOXO activity. Both JNK-1 and DAF-16 appeared to act within neurons in a cell-autonomous manner to influence branching, and, through their tissue-specific expression, it was possible to disconnect the rate at which branching occurred from the overall rate of aging of the animal. Old age has generally been associated with the decline and deterioration of different tissues, except in the case of tumor cell growth. To our knowledge, this is the first indication that aging can potentiate another form of growth, the growth of neurite branches, in normal animals.
Figures


References
-
- Adachi H, Fujiwara Y, Ishii N. Effects of oxygen on protein carbonyl and aging in Caenorhabditis elegans mutants with long (age-1) and short (mev-1) life spans. J Gerontol A Biol Sci Med Sci. 1998;53:B240–B244. - PubMed
-
- Björkblom B, Ostman N, Hongisto V, Komarovski V, Filén JJ, Nyman TA, Kallunki T, Courtney MJ, Coffey ET. Constitutively active cytoplasmic c-Jun N-terminal kinase 1 is a dominant regulator of dendritic architecture: role of microtubule-associated protein 2 as an effector. J Neurosci. 2005;25:6350–6361. - PMC - PubMed
-
- Braeckman BP, Houthoofd K, De Vreese A, Vanfleteren JR. Apparent uncoupling of energy production and consumption in long-lived Clk mutants of Caenorhabditis elegans. Curr Biol. 1999;9:493–496. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
