The novel costimulatory programmed death ligand 1/B7.1 pathway is functional in inhibiting alloimmune responses in vivo
- PMID: 21697455
- PMCID: PMC3140607
- DOI: 10.4049/jimmunol.1100056
The novel costimulatory programmed death ligand 1/B7.1 pathway is functional in inhibiting alloimmune responses in vivo
Abstract
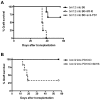
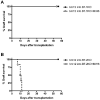
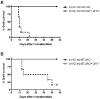
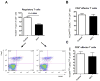
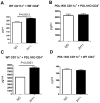
The programmed death ligand 1 (PDL1)/programmed death 1 (PD1) costimulatory pathway plays an important role in the inhibition of alloimmune responses as well as in the induction and maintenance of peripheral tolerance. It has been demonstrated recently that PDL1 also can bind B7.1 to inhibit T cell responses in vitro. Using the bm12 into B6 heart transplant model, we investigated the functional significance of this interaction in alloimmune responses in vivo. PD1 blockade unlike PDL1 blockade failed to accelerate bm12 allograft rejection, suggesting a role for an additional binding partner for PDL1 other than PD1 in transplant rejection. PDL1 blockade was able to accelerate allograft rejection in B7.2-deficient recipients but not B7.1-deficient recipients, indicating that PDL1 interaction with B7.1 was important in inhibiting rejection. Administration of the novel 2H11 anti-PDL1 mAb, which only blocks the PDL1-B7.1 interaction, aggravated chronic injury of bm12 allografts in B6 recipients. Aggravated chronic injury was associated with an increased frequency of alloreactive IFN-γ-, IL-4-, and IL-6-producing splenocytes and a decreased percentage of regulatory T cells in the recipients. Using an in vitro cell culture assay, blockade of the interaction of PDL1 on dendritic cells with B7.1 on T cells increased IFN-γ production from alloreactive CD4(+) T cells, whereas blockade of dendritic cell B7.1 interaction with T cell PDL1 did not. These data indicate that PDL1 interaction with B7.1 plays an important role in the inhibition of alloimmune responses in vivo and suggests a dominant direction for PDL1 and B7.1 interaction.
Conflict of interest statement
Figures

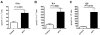
References
-
- Dong VM, Womer KL, Sayegh MH. Transplantation tolerance: the concept and its applicability. Pediatr Transplant. 1999;3:181–192. - PubMed
-
- Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. - PMC - PubMed
-
- Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. - PubMed
-
- Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

