Binding of the mannose-specific lectin, griffithsin, to HIV-1 gp120 exposes the CD4-binding site
- PMID: 21697467
- PMCID: PMC3165825
- DOI: 10.1128/JVI.02675-10
Binding of the mannose-specific lectin, griffithsin, to HIV-1 gp120 exposes the CD4-binding site
Abstract
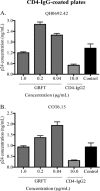
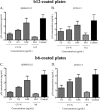
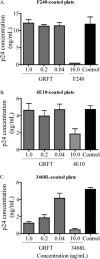
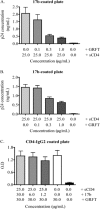
The glycans on HIV-1 gp120 play an important role in shielding neutralization-sensitive epitopes from antibody recognition. They also serve as targets for lectins that bind mannose-rich glycans. In this study, we investigated the interaction of the lectin griffithsin (GRFT) with HIV-1 gp120 and its effects on exposure of the CD4-binding site (CD4bs). We found that GRFT enhanced the binding of HIV-1 to plates coated with anti-CD4bs antibodies b12 and b6 or the CD4 receptor mimetic CD4-IgG2. The average enhancement of b12 or b6 binding was higher for subtype B viruses than for subtype C, while for CD4-IgG2, it was similar for both subtypes, although lower than observed with antibodies. This GRFT-mediated enhancement of HIV-1 binding to b12 was reflected in synergistic neutralization for 2 of the 4 viruses tested. The glycan at position 386, which shields the CD4bs, was involved in both GRFT-mediated enhancement of binding and neutralization synergism between GRFT and b12. Although GRFT enhanced CD4bs exposure, it simultaneously inhibited ligand binding to the coreceptor binding site, suggesting that GRFT-dependent enhancement and neutralization utilize independent mechanisms. This study shows for the first time that GRFT interaction with gp120 exposes the CD4bs through binding the glycan at position 386, which may have implications for how to access this conserved site.
Figures


References
-
- Balzarini J. 2005. Targeting the glycans of gp120: a novel approach aimed at the Achilles heel of HIV. Lancet Infect. Dis. 5:726–731 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

