Arp2/3 promotes junction formation and maintenance in the Caenorhabditis elegans intestine by regulating membrane association of apical proteins
- PMID: 21697505
- PMCID: PMC3154884
- DOI: 10.1091/mbc.E10-10-0862
Arp2/3 promotes junction formation and maintenance in the Caenorhabditis elegans intestine by regulating membrane association of apical proteins
Abstract
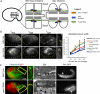
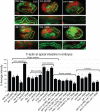
It has been proposed that Arp2/3, which promotes nucleation of branched actin, is needed for epithelial junction initiation but is less important as junctions mature. We focus here on how Arp2/3 contributes to the Caenorhabditis elegans intestinal epithelium and find important roles for Arp2/3 in the maturation and maintenance of junctions in embryos and adults. Electron microscope studies show that embryos depleted of Arp2/3 form apical actin-rich microvilli and electron-dense apical junctions. However, whereas apical/basal polarity initiates, apical maturation is defective, including decreased apical F-actin enrichment, aberrant lumen morphology, and reduced accumulation of some apical junctional proteins, including DLG-1. Depletion of Arp2/3 in adult animals leads to similar intestinal defects. The DLG-1/AJM-1 apical junction proteins, and the ezrin-radixin-moesin homologue ERM-1, a protein that connects F-actin to membranes, are required along with Arp2/3 for apical F-actin enrichment in embryos, whereas cadherin junction proteins are not. Arp2/3 affects the subcellular distribution of DLG-1 and ERM-1. Loss of Arp2/3 shifts both ERM-1 and DLG-1 from pellet fractions to supernatant fractions, suggesting a role for Arp2/3 in the distribution of membrane-associated proteins. Thus, Arp2/3 is required as junctions mature to maintain apical proteins associated with the correct membranes.
Figures




References
-
- Allen RD, Wolf RW. Membrane recycling at the cytoproct of Tetrahymena. J Cell Sci. 1979;35:217–227. - PubMed
-
- Bossinger O, Klebes A, Segbert C, Theres C, Knust E. Zonula adherens formation in Caenorhabditis elegans requires dlg-1, the homologue of the Drosophila gene discs large. Dev Biol. 2001;230:29–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

