Regioselective reactions for programmable resveratrol oligomer synthesis
- PMID: 21697944
- PMCID: PMC3179663
- DOI: 10.1038/nature10197
Regioselective reactions for programmable resveratrol oligomer synthesis
Abstract
Although much attention has been devoted to resveratrol, a unique polyphenol produced by plants and credited as potentially being responsible for the 'French paradox'--the observation that French people have a relatively low incidence of coronary heart disease, even though their diet is high in saturated fats--the oligomers of resveratrol have been largely ignored despite their high biological activity. Challenges in achieving their isolation in sufficient quantity from natural sources, coupled with an inability to prepare them easily synthetically, are seen as the main obstacles. Here we report a programmable, controlled and potentially scalable synthesis of the resveratrol family via a three-stage design. The synthetic approach requires strategy- and reagent-guided chemical functionalizations to differentiate two distinct cores possessing multiple sites with the same or similar reactivity, ultimately leading to five higher-order natural products. This work demonstrates that challenging, positionally selective functionalizations of complex materials are possible where biosynthetic studies have indicated otherwise, it provides materials and tools with which to unlock the full biochemical potential of this family of natural products, and it affords an intellectual framework within which other oligomeric families could potentially be accessed.
Figures
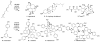



Comment in
-
Organic chemistry: Triumph for unnatural synthesis.Nature. 2011 Jun 22;474(7352):459-60. doi: 10.1038/474459a. Nature. 2011. PMID: 21697943 No abstract available.
References
-
- Fischbach MA, Clardy J. One pathway, many products. Nature: Chem. Biol. 2007;3:353–355. - PubMed
-
- Christianson DW. Structural biology and chemistry of the terpenoid cyclases. Chem. Rev. 2006;106:3412–3442. - PubMed
-
- Chen K, Baran PS. Total synthesis of eudesmane terpenes by site-selective C–H oxidations. Nature. 2009;459:824–828. - PubMed
-
- Sotheeswaran S, Pasupathy V. Distribution of resveratrol oligomers in plants. Phytochemistry. 1993;32:1083–1092.
-
- Quideau S, Deffieux D, Douat-Casassus C, Pouységu L. Plant polyphenols: chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011;50:586–621. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

