New therapeutic targets in Alzheimer's disease: brain deregulation of calcium and zinc
- PMID: 21697951
- PMCID: PMC3168999
- DOI: 10.1038/cddis.2011.57
New therapeutic targets in Alzheimer's disease: brain deregulation of calcium and zinc
Abstract
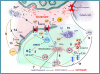
The molecular determinants of Alzheimer's (AD) disease are still not completely known; however, in the past two decades, a large body of evidence has indicated that an important contributing factor for the disease is the development of an unbalanced homeostasis of two signaling cations: calcium (Ca(2+)) and zinc (Zn(2+)). Both ions serve a critical role in the physiological functioning of the central nervous system, but their brain deregulation promotes amyloid-β dysmetabolism as well as tau phosphorylation. AD is also characterized by an altered glutamatergic activation, and glutamate can promote both Ca(2+) and Zn(2+) dyshomeostasis. The two cations can operate synergistically to promote the generation of free radicals that further intracellular Ca(2+) and Zn(2+) rises and set the stage for a self-perpetuating harmful loop. These phenomena can be the initial steps in the pathogenic cascade leading to AD, therefore, therapeutic interventions aiming at preventing Ca(2+) and Zn(2+) dyshomeostasis may offer a great opportunity for disease-modifying strategies.
Figures



References
-
- Sensi SL, Paoletti P, Bush AI, Sekler I. Zinc in the physiology and pathology of the CNS. Nat Rev Neurosci. 2009;10:780–791. - PubMed
-
- Green KN, LaFerla FM. Linking calcium to Abeta and Alzheimer's disease. Neuron. 2008;59:190–194. - PubMed
-
- Lannfelt L, Blennow K, Zetterberg H, Batsman S, Ames D, Harrison J, et al. Safety, efficacy, and biomarker findings of PBT2 in targeting Abeta as a modifying therapy for Alzheimer's disease: a phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2008;7:779–786. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

