Total retinal blood flow measurement with ultrahigh speed swept source/Fourier domain OCT
- PMID: 21698017
- PMCID: PMC3114222
- DOI: 10.1364/BOE.2.001539
Total retinal blood flow measurement with ultrahigh speed swept source/Fourier domain OCT
Abstract
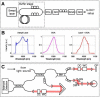
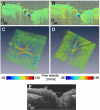
Doppler OCT provides depth-resolved information on flow in biological tissues. In this article, we demonstrate ultrahigh speed swept source/Fourier domain OCT for visualization and quantitative assessment of retinal blood flow. Using swept laser technology, the system operated in the 1050-nm wavelength range at a high axial scan rate of 200 kHz. The rapid imaging speed not only enables volumetric imaging with high axial scan densities, but also enables measurement of high flow velocities in the central retinal vessels. Deep penetration in the optic nerve and lamina cribrosa was achieved by imaging at 1-µm wavelengths. By analyzing en-face images extracted from 3D Doppler data sets, absolute flow in single vessels as well as total retinal blood flow was measured using a simple and robust protocol that does not require measurement of Doppler angles. The results from measurements in healthy eyes suggest that ultrahigh speed swept source/Fourier domain OCT could be a promising technique for volumetric imaging of retinal vasculature and quantitation of retinal blood flow in a wide range of retinal diseases.
Keywords: (170.3880) Medical and biological imaging; (170.4470) Ophthalmology; (170.4500) Optical coherence tomography; (280.2490) Flow diagnostics.
Figures




References
-
- Friedman E., “A hemodynamic model of the pathogenesis of age-related macular degeneration,” Am. J. Ophthalmol. 124(5), 677–682 (1997). - PubMed
-
- Lieb W. E., Cohen S. M., Merton D. A., Shields J. A., Mitchell D. G., Goldberg B. B., “Color Doppler imaging of the eye and orbit. Technique and normal vascular anatomy,” Arch. Ophthalmol. 109(4), 527–531 (1991). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
