Photodynamic antimicrobial chemotherapy in aquaculture: photoinactivation studies of Vibrio fischeri
- PMID: 21698119
- PMCID: PMC3117864
- DOI: 10.1371/journal.pone.0020970
Photodynamic antimicrobial chemotherapy in aquaculture: photoinactivation studies of Vibrio fischeri
Abstract
Background: Photodynamic antimicrobial chemotherapy (PACT) combines light, a light-absorbing molecule that initiates a photochemical or photophysical reaction, and oxygen. The combined action of these three components originates reactive oxygen species that lead to microorganisms' destruction. The aim was to evaluate the efficiency of PACT on Vibrio fischeri: 1) with buffer solution, varying temperature, pH, salinity and oxygen concentration values; 2) with aquaculture water, to reproduce photoinactivation (PI) conditions in situ.
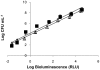
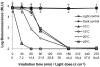
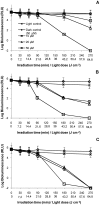
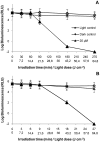
Methodology/principal findings: To monitor the PI kinetics, the bioluminescence of V. fischeri was measured during the experiments. A tricationic meso-substituted porphyrin (Tri-Py(+)-Me-PF) was used as photosensitizer (5 µM in the studies with buffer solution and 10-50 µM in the studies with aquaculture water); artificial white light (4 mW cm(-2)) and solar irradiation (40 mW cm(-2)) were used as light sources; and the bacterial concentration used for all experiments was ≈10(7) CFU mL(-1) (corresponding to a bioluminescence level of 10(5) relative light units--RLU). The variations in pH (6.5-8.5), temperature (10-25°C), salinity (20-40 g L(-1)) and oxygen concentration did not significantly affect the PI of V. fischeri, once in all tested conditions the bioluminescent signal decreased to the detection limit of the method (≈7 log reduction). The assays using aquaculture water showed that the efficiency of the process is affected by the suspended matter. Total PI of V. fischeri in aquaculture water was achieved under solar light in the presence of 20 µM of Tri-Py(+)-Me-PF.
Conclusions/significance: If PACT is to be used in environmental applications, the matrix containing target microbial communities should be previously characterized in order to establish an efficient protocol having into account the photosensitizer concentration, the light source and the total light dose delivered. The possibility of using solar light in PACT to treat aquaculture water makes this technology cost-effective and attractive.
Conflict of interest statement
Figures




References
-
- Olafsen JA. Interactions between fish larvae and bacteria in marine aquaculture. Aquaculture. 2001;200:223–247.
-
- Muroga K. Viral and bacterial diseases of marine fish and shellfish in Japanese hatcheries. Aquaculture. 2001;202:23–44.
-
- Defoirdt T, Boon N, Sorgeloos P, Verstraete W, Bossier P. Alternatives to antibiotics to control bacterial infections: luminescent vibriosis in aquaculture as an example. Trends Biotechnol. 2007;25:472–479. - PubMed
-
- Moriarty DJW. Control of luminous Vibrio species in penaeid aquaculture ponds. Aquaculture. 1998;164:351–358.
-
- Moriarty DJW. Disease control in shrimp aquaculture with probiotic bacteria. Microbial biosystems: new frontiers. In: Bell CR, Brylinsky M, Johnson-Green P, editors. Proceeding of the 8th International Symposium on Microbial Ecology. Canada: Microbial Interactions in Aquaculture; 1999.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

