C-terminus of progranulin interacts with the beta-propeller region of sortilin to regulate progranulin trafficking
- PMID: 21698296
- PMCID: PMC3115958
- DOI: 10.1371/journal.pone.0021023
C-terminus of progranulin interacts with the beta-propeller region of sortilin to regulate progranulin trafficking
Abstract
Progranulin haplo-insufficiency is a main cause of frontotemporal lobar degeneration (FTLD) with TDP-43 aggregates. Previous studies have shown that sortilin regulates progranulin trafficking and is a main determinant of progranulin level in the brain. In this study, we mapped the binding site between progranulin and sortilin. Progranulin binds to the beta-propeller region of sortilin through its C-terminal tail. The C-terminal progranulin fragment is fully sufficient for sortilin binding and progranulin C-terminal peptide displaces progranulin binding to sortilin. Deletion of the last 3 residues of progranulin (QLL) abolishes its binding to sortilin and also sortilin dependent regulation of progranulin trafficking. Since progranulin haplo-insufficiency results in FTLD, these results may provide important insights into future studies of progranulin trafficking and signaling and progranulin based therapy for FTLD.
Conflict of interest statement
Figures

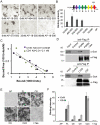
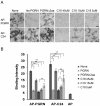
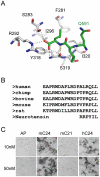
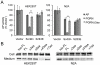
References
-
- Neary D, Snowden J, Mann D. Frontotemporal dementia. Lancet Neurol. 2005;4:771–780. - PubMed
-
- Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology. 2002;58:1615–1621. - PubMed
-
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. - PubMed
-
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. - PubMed
-
- Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442:920–924. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

