Minutissamides A-D, antiproliferative cyclic decapeptides from the cultured cyanobacterium Anabaena minutissima
- PMID: 21699148
- PMCID: PMC3142320
- DOI: 10.1021/np2002226
Minutissamides A-D, antiproliferative cyclic decapeptides from the cultured cyanobacterium Anabaena minutissima
Abstract
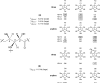
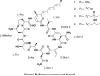
Four cyclic decapeptides, minutissamides A-D (1-4), were isolated from the cultured cyanobacterium Anabaena minutissima (UTEX 1613). The planar structures were determined using various spectroscopic techniques including HRESIMS and 1D and 2D NMR experiments. The absolute configurations of the α-amino acid residues were assigned using Marfey's method after acid hydrolysis. The absolute configuration of a β-amino acid residue was assigned by a combination of the advanced Marfey's method, J-based configurational analysis, and ROE spectroscopic analysis. The structures of minutissamides A-D (1-4) were characterized by the presence of three nonstandard α-amino acid residues (two α,β-dehydro-α-aminobutyric acids and one N-methylated Asn) and one β-amino acid residue (2-hydroxy-3-amino-4-methyldodecanoic acid or 2-hydroxy-3-amino-4-methylhexadecanoic acid). Minutissamides A-D (1-4) exhibited antiproliferative activity against the HT-29 human colon cancer cell line with IC₅₀ values of 2.0, 20.0, 11.8, and 22.7 μM, respectively.
Figures


References
-
- Tan LT. Phytochemistry. 2007;68:954–979. - PubMed
-
- Wagoner RMV, Drummond AK, Wright JL. C. Adv. Appl. Microbiol. 2007;61:89–217. - PubMed
-
- Harada KI. Chem. Pharm. Bull. 2004;52:889–899. - PubMed
-
- Singh S, Kate BN, Banerjee UC. Critical. Rev. Biotech. 2005;25:73–95. - PubMed
-
- Burja AM, Banaigs B, Abou-Mansour E, Burgess JG, Wright PC. Tetrahedron. 2001;57:9347–9377.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

