Proteolysis in illness-associated skeletal muscle atrophy: from pathways to networks
- PMID: 21699435
- PMCID: PMC5734931
- DOI: 10.3109/10408363.2011.586171
Proteolysis in illness-associated skeletal muscle atrophy: from pathways to networks
Abstract
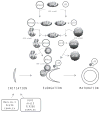
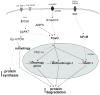
Improvements in health in the past decades have resulted in increased numbers of the elderly in both developed and developing regions of the world. Advances in therapy have also increased the prevalence of patients with chronic and degenerative diseases. Muscle wasting, a feature of most chronic diseases, is prominent in the elderly and contributes to both morbidity and mortality. A major research goal has been to identify the proteolytic system(s) that is responsible for the degradation of proteins that occurs in muscle atrophy. Findings over the past 20 years have clearly confirmed an important role of the ubiquitin proteasome system in mediating muscle proteolysis, particularly that of myofibrillar proteins. However, recent observations have provided evidence that autophagy, calpains and caspases also contribute to the turnover of muscle proteins in catabolic states, and furthermore, that these diverse proteolytic systems interact with each other at various levels. Importantly, a number of intracellular signaling pathways such as the IGF1/AKT, myostatin/Smad, PGC1, cytokine/NFκB, and AMPK pathways are now known to interact and can regulate some of these proteolytic systems in a coordinated manner. A number of loss of function studies have identified promising therapeutic approaches to the prevention and treatment of wasting. However, additional biomarkers and other approaches to improve early identification of patients who would benefit from such treatment need to be developed. The current data suggests a network of interacting proteolytic and signaling pathways in muscle. Future studies are needed to improve understanding of the nature and control of these interactions and how they work to preserve muscle function under various states of growth and atrophy.
Conflict of interest statement
The authors report no declarations of interest.
Figures

References
-
- Trends in aging - United States and worldwide. CDC. MMWR Morb Mortal Wkly Rep. 2003;52:101–104. 106. - PubMed
-
- Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793–799. - PubMed
-
- Marquis K, Debigaré R, Lacasse Y, LeBlanc P, Jobin J, Carrier G, et al. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Amer J Resp Crit Care. 2002;166:809–813. - PubMed
-
- Anker SD, Coats AJ. Cardiac cachexia: a syndrome with impaired survival and immune and neuroendocrine activation. Chest. 1999;115:836–847. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
