Myelin associated inhibitors: a link between injury-induced and experience-dependent plasticity
- PMID: 21699896
- PMCID: PMC3189418
- DOI: 10.1016/j.expneurol.2011.06.006
Myelin associated inhibitors: a link between injury-induced and experience-dependent plasticity
Abstract
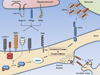
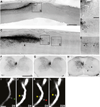
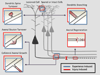
In the adult, both neurologic recovery and anatomical growth after a CNS injury are limited. Two classes of growth inhibitors, myelin associated inhibitors (MAIs) and extracellular matrix associated inhibitors, limit both functional recovery and anatomical rearrangements in animal models of spinal cord injury. Here we focus on how MAIs limit a wide spectrum of growth that includes regeneration, sprouting, and plasticity in both the intact and lesioned CNS. Three classic myelin associated inhibitors, Nogo-A, MAG, and OMgp, signal through their common receptors, Nogo-66 Receptor-1 (NgR1) and Paired-Immunoglobulin-like-Receptor-B (PirB), to regulate cytoskeletal dynamics and inhibit growth. Initially described as inhibitors of axonal regeneration, subsequent work has demonstrated that MAIs also limit activity and experience-dependent plasticity in the intact, adult CNS. MAIs therefore represent a point of convergence for plasticity that limits anatomical rearrangements regardless of the inciting stimulus, blurring the distinction between injury studies and more "basic" plasticity studies.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
References
-
- Burns SP, et al. Recovery of ambulation in motor-incomplete tetraplegia. Archives of Physical Medicine and Rehabilitation. 1997;78(11):1169–1172. - PubMed
-
- Geisler FHC, William P, Giacinto Grieco, Devinder Poonian the Sygen Study Group. Measurements and Recovery Patterns in a Multicenter Study of Acute Spinal Cord Injury. Spine. 2001;26(24S):S68–S86. - PubMed
-
- Cajal SRy. Cajal's Degeneration and Regeneration of the Nervous System. Oxford Univeristy Press; 1991. p. 976.
-
- David S, Aguayo A. Axonal elongation into peripheral nervous system "bridges" after central nervous system injury in adult rats. Science. 1981;214(4523):931–933. - PubMed
-
- Richardson PM, McGuinness UM, Aguayo AJ. Axons from CNS neurones regenerate into PNS grafts. Nature. 1980;284(5753):264–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

