Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies
- PMID: 21700325
- PMCID: PMC3132082
- DOI: 10.1016/j.cell.2011.06.001
Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies
Abstract
Parkinson's disease (PD), an adult neurodegenerative disorder, has been clinically linked to the lysosomal storage disorder Gaucher disease (GD), but the mechanistic connection is not known. Here, we show that functional loss of GD-linked glucocerebrosidase (GCase) in primary cultures or human iPS neurons compromises lysosomal protein degradation, causes accumulation of α-synuclein (α-syn), and results in neurotoxicity through aggregation-dependent mechanisms. Glucosylceramide (GlcCer), the GCase substrate, directly influenced amyloid formation of purified α-syn by stabilizing soluble oligomeric intermediates. We further demonstrate that α-syn inhibits the lysosomal activity of normal GCase in neurons and idiopathic PD brain, suggesting that GCase depletion contributes to the pathogenesis of sporadic synucleinopathies. These findings suggest that the bidirectional effect of α-syn and GCase forms a positive feedback loop that may lead to a self-propagating disease. Therefore, improved targeting of GCase to lysosomes may represent a specific therapeutic approach for PD and other synucleinopathies.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
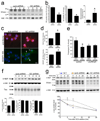
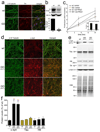
 p<0.01 compared to wt α-syn, wt and GD tau, and wt and GD Htt; *p<0.05 compared to wt tau).
p<0.01 compared to wt α-syn, wt and GD tau, and wt and GD Htt; *p<0.05 compared to wt tau).
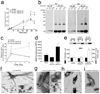
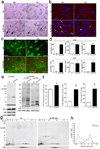


Comment in
-
A feedforward loop links Gaucher and Parkinson's diseases?Cell. 2011 Jul 8;146(1):9-11. doi: 10.1016/j.cell.2011.06.031. Cell. 2011. PMID: 21729776
-
Looping the link between Gaucher and Parkinson's disease.Clin Genet. 2011 Nov;80(5):426-7. doi: 10.1111/j.1399-0004.2011.01768.x. Clin Genet. 2011. PMID: 21883165 No abstract available.
-
Links between glucocerebrosidase and alpha-synuclein revealed.Mov Disord. 2011 Oct;26(12):2177. doi: 10.1002/mds.23985. Mov Disord. 2011. PMID: 22319791 No abstract available.
References
-
- Auluck PK, Chan HY, Trojanowski JQ, Lee VM, Bonini NM. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson's disease. Science. 2002;295:865–868. - PubMed
-
- Brady RO, Kanfer J, Shapiro D. The Metabolism of Glucocerebrosides. I. Purification and Properties of a Glucocerebroside-Cleaving Enzyme from Spleen Tissue. J Biol Chem. 1965;240:39–43. - PubMed
-
- Chandra S, Gallardo G, Fernandez-Chacon R, Schluter OM, Sudhof TC. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–396. - PubMed
-
- Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4:1318–1320. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

