Acid ceramidase expression modulates the sensitivity of A375 melanoma cells to dacarbazine
- PMID: 21700700
- PMCID: PMC3151065
- DOI: 10.1074/jbc.M110.216382
Acid ceramidase expression modulates the sensitivity of A375 melanoma cells to dacarbazine
Abstract
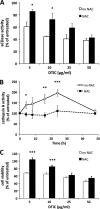
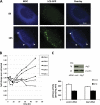
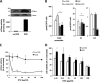
Dacarbazine (DTIC) is the treatment of choice for metastatic melanoma, but its response in patients remains very poor. Ceramide has been shown to be a death effector and to play an important role in regulating cancer cell growth upon chemotherapy. Among ceramidases, the enzymes that catabolize ceramide, acid ceramidase (aCDase) has been implicated in cancer progression. Here we show that DTIC elicits a time- and dose-dependent decrease of aCDase activity and an increase of intracellular ceramide levels in human A375 melanoma cells. The loss of enzyme activity occurred as a consequence of reactive oxygen species-dependent activation of cathepsin B-mediated degradation of aCDase. These events preceded autophagic features and loss of cell viability. Down-regulation of acid but not neutral or alkaline ceramidase 2 resulted in elevated levels of ceramide and sensitization to the toxic effects of DTIC. Conversely, inducible overexpression of acid but not neutral ceramidase reduced ceramide levels and conferred resistance to DTIC. In conclusion, we report that increased levels of ceramide, due to enhanced degradation of aCDase, are in part responsible for the cell death effects of DTIC. These results suggest that down-regulation of aCDase alone or in combination with DTIC may represent a useful tool in the treatment of metastatic melanoma.
Figures
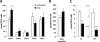


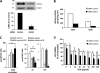
References
-
- Gandini S., Sera F., Cattaruzza M. S., Pasquini P., Zanetti R., Masini C., Boyle P., Melchi C. F. (2005) Eur. J. Cancer 41, 2040–2059 - PubMed
-
- Jemal A., Siegel R., Ward E., Hao Y., Xu J., Murray T., Thun M. J. (2008) CA Cancer J. Clin. 58, 71–96 - PubMed
-
- Mouawad R., Sebert M., Michels J., Bloch J., Spano J. P., Khayat D. (2010) Crit. Rev. Oncol. Hematol. 74, 27–39 - PubMed
-
- Middleton M. R., Grob J. J., Aaronson N., Fierlbeck G., Tilgen W., Seiter S., Gore M., Aamdal S., Cebon J., Coates A., Dreno B., Henz M., Schadendorf D., Kapp A., Weiss J., Fraass U., Statkevich P., Muller M., Thatcher N. (2000) J. Clin. Oncol. 18, 158–166 - PubMed
-
- Ogretmen B., Hannun Y. A. (2004) Nat. Rev. Cancer 4, 604–616 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

