A pilot study of subcutaneous decitabine in β-thalassemia intermedia
- PMID: 21700776
- PMCID: PMC3172790
- DOI: 10.1182/blood-2011-03-341909
A pilot study of subcutaneous decitabine in β-thalassemia intermedia
Abstract
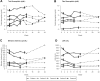
Ineffective erythropoiesis, the hallmark of β-thalassemia, is a result of α/non-α globin chain imbalance. One strategy to redress globin-chain imbalance is to induce γ-globin gene (HBG) expression. Repression of HBG in adult erythroid cells involves DNA methylation and other epigenetic changes. Therefore, the cytosine analog decitabine, which can deplete DNA methyltransferase 1 (DNMT1), can potentially activate HBG. In 5 patients with β-thalassemia intermedia, a dose and schedule of decitabine intended to deplete DNMT1 without causing significant cytotoxicity (0.2 mg/kg subcutaneous 2 times per week for 12 weeks) increased total hemoglobin from 7.88 ± 0.88 g/dL to 9.04 ± 0.77 g/dL (P = .004) and absolute fetal hemoglobin from 3.64 ± 1.13 g/dL to 4.29 ± 1.13 g/dL (P = .003). Significant favorable changes also occurred in indices of hemolysis and red blood cell densitometry. Consistent with a noncytotoxic, differentiation altering mechanism of action, the major side effect was an asymptomatic increase in platelet counts without erythrocyte micronucleus or VDJ recombination assay evidence of genotoxicity. This study was registered at www.clinicaltrials.gov as #NCT00661726.
Figures
References
-
- Wood WG, Weatherall DJ, Clegg JB. Interaction of heterocellular hereditary persistence of foetal haemoglobin with beta thalassaemia and sickle cell anaemia. Nature. 1976;264(5583):247–249. - PubMed
-
- Taher AT, Musallam KM, Karimi M, et al. Overview on practices in thalassemia intermedia management aiming for lowering complication rates across a region of endemicity: the OPTIMAL CARE study. Blood. 2010;115(10):1886–1892. - PubMed
-
- Saunthararajah Y, Hillery CA, Lavelle D, et al. Effects of 5-aza-2′-deoxycytidine on fetal hemoglobin levels, red cell adhesion, and hematopoietic differentiation in patients with sickle cell disease. Blood. 2003;102(12):3865–3870. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

