Specific effects of BCL10 Serine mutations on phosphorylations in canonical and noncanonical pathways of NF-κB activation following carrageenan
- PMID: 21700900
- PMCID: PMC3174537
- DOI: 10.1152/ajpgi.00071.2011
Specific effects of BCL10 Serine mutations on phosphorylations in canonical and noncanonical pathways of NF-κB activation following carrageenan
Abstract
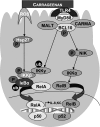
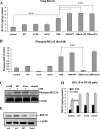
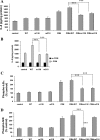
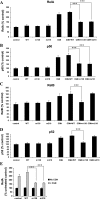
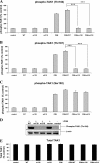
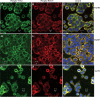
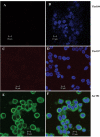
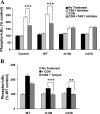
To determine the impact of B cell leukemia/lymphoma (BCL) 10 on the phosphorylation of crucial mediators in NF-κB-mediated inflammatory pathways, human colonic epithelial cells were exposed to carrageenan (CGN), a sulfated polysaccharide commonly used as a food additive and known to induce NF-κB nuclear translocation by both canonical and noncanonical pathways. Phosphorylations of intermediates in inflammatory cascades, including NF-κB-inducing kinase (NIK) at Thr(559), transforming growth factor-β-activating kinase (TAK) 1 at Thr(184), Thr(187), and Ser(192), and inhibitory factor κBα (IκBα) at Ser(32), were examined following mutation of BCL10 at Ser(138) and at Ser(218). Specific phosphoantibodies were used for detection by enzyme-linked immunosorbent assay, immunoblot, and confocal microscopy of differences in phosphorylation following transfection by mutated BCL10. Both mutations demonstrated dominant-negative effects, with inhibition of phospho(Ser(32))-IκBα to less than control levels. Both of the BCL10 mutations reduced the CGN-induced increases in nuclear RelA and p50, but only the Ser(138) mutation inhibited the CGN-induced increases in nuclear RelB and p52 and in NIK Thr(559) phosphorylation. Hence, the phosphorylation of BCL10 Ser(138), but not Ser(218), emerged as a critical event in activation of the noncanonical pathway of NF-κB activation. Either BCL10 Ser(138) or Ser(218) mutation inhibited the phosphorylation of TAK1 at Thr(184) and at Thr(187), but not at Ser(192). These findings indicate that BCL10 phosphorylations act upstream of phosphorylations of NIK, TAK1, and IκBα and differentially affect the canonical and noncanonical pathways of NF-κB activation.
Figures
Similar articles
-
B-cell CLL/lymphoma 10 (BCL10) is required for NF-kappaB production by both canonical and noncanonical pathways and for NF-kappaB-inducing kinase (NIK) phosphorylation.J Biol Chem. 2010 Jan 1;285(1):522-30. doi: 10.1074/jbc.M109.050815. Epub 2009 Nov 6. J Biol Chem. 2010. PMID: 19897484 Free PMC article.
-
Lipopolysaccharide-induced activation of NF-κB non-canonical pathway requires BCL10 serine 138 and NIK phosphorylations.Exp Cell Res. 2010 Nov 15;316(19):3317-27. doi: 10.1016/j.yexcr.2010.05.004. Epub 2010 May 11. Exp Cell Res. 2010. PMID: 20466000 Free PMC article.
-
Prolongation of carrageenan-induced inflammation in human colonic epithelial cells by activation of an NFκB-BCL10 loop.Biochim Biophys Acta. 2012 Aug;1822(8):1300-7. doi: 10.1016/j.bbadis.2012.05.001. Epub 2012 May 8. Biochim Biophys Acta. 2012. PMID: 22579587 Free PMC article.
-
The noncanonical NF-κB pathway.Immunol Rev. 2012 Mar;246(1):125-40. doi: 10.1111/j.1600-065X.2011.01088.x. Immunol Rev. 2012. PMID: 22435551 Free PMC article. Review.
-
Biochemical and Cellular Profile of NIK Inhibitors with Long Residence Times.SLAS Discov. 2021 Jun;26(5):676-683. doi: 10.1177/2472555220964450. Epub 2020 Oct 21. SLAS Discov. 2021. PMID: 33084478 Review.
Cited by
-
The Role of Carrageenan in Inflammatory Bowel Diseases and Allergic Reactions: Where Do We Stand?Nutrients. 2021 Sep 27;13(10):3402. doi: 10.3390/nu13103402. Nutrients. 2021. PMID: 34684400 Free PMC article. Review.
-
Carrageenan-induced colonic inflammation is reduced in Bcl10 null mice and increased in IL-10-deficient mice.Mediators Inflamm. 2013;2013:397642. doi: 10.1155/2013/397642. Epub 2013 May 26. Mediators Inflamm. 2013. PMID: 23766559 Free PMC article.
-
A Critical Role for Dopamine D5 Receptors in Pain Chronicity in Male Mice.J Neurosci. 2018 Jan 10;38(2):379-397. doi: 10.1523/JNEUROSCI.2110-17.2017. Epub 2017 Nov 22. J Neurosci. 2018. PMID: 29167404 Free PMC article.
-
Maprotiline inhibits COX2 and iNOS gene expression in lipopolysaccharide-stimulated U937 macrophages and carrageenan-induced paw edema in rats.Cent Eur J Immunol. 2019;44(1):15-22. doi: 10.5114/ceji.2019.84011. Epub 2019 Apr 15. Cent Eur J Immunol. 2019. PMID: 31114432 Free PMC article.
-
Elevated TAK1 augments tumor growth and metastatic capacities of ovarian cancer cells through activation of NF-κB signaling.Oncotarget. 2014 Sep 15;5(17):7549-62. doi: 10.18632/oncotarget.2273. Oncotarget. 2014. PMID: 25277189 Free PMC article.
References
-
- Bhattacharyya S, Borthakur A, Pant N, Dudeja PK, Tobacman JK. Bcl10 mediates LPS-induced activation of NF-kappaB and IL-8 in human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 293: G429–G437, 2007 - PubMed
-
- Bhattacharyya S, Dudeja PK, Tobacman JK. Lipopolysaccharide activates NF-kappaB by TLR4-Bcl10-dependent and independent pathways in colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol 295: G784–G790, 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

