Transglutaminase 2 and its role in pulmonary fibrosis
- PMID: 21700912
- PMCID: PMC3208598
- DOI: 10.1164/rccm.201101-0013OC
Transglutaminase 2 and its role in pulmonary fibrosis
Abstract
Rationale: Idiopathic pulmonary fibrosis (IPF) is a deadly progressive disease with few treatment options. Transglutaminase 2 (TG2) is a multifunctional protein, but its function in pulmonary fibrosis is unknown.
Objectives: To determine the role of TG2 in pulmonary fibrosis.
Methods: The fibrotic response to bleomycin was compared between wild-type and TG2 knockout mice. Transglutaminase and transglutaminase-catalyzed isopeptide bond expression was examined in formalin-fixed human lung biopsy sections by immunohistochemistry from patients with IPF. In addition, primary human lung fibroblasts were used to study TG2 function in vitro.
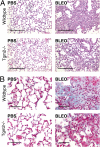
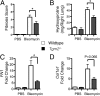
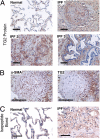
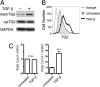
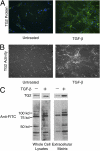
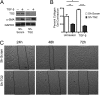
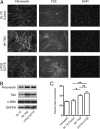
Measurements and main results: TG2 knockout mice developed significantly reduced fibrosis compared with wild-type mice as determined by hydroxyproline content and histologic fibrosis score (P < 0.05). TG2 expression and activity are increased in lung biopsy sections in humans with IPF compared with normal control subjects. In vitro overexpression of TG2 led to increased fibronectin deposition, whereas transglutaminase knockdown led to defects in contraction and adhesion. The profibrotic cytokine transforming growth factor-β causes an increase in membrane-localized TG2, increasing its enzymatic activity.
Conclusions: TG2 is involved in pulmonary fibrosis in a mouse model and in human disease and is important in normal fibroblast function. With continued research on TG2, it may offer a new therapeutic target.
Figures

Comment in
-
Idiopathic pulmonary fibrosis: the matrix is the message.Am J Respir Crit Care Med. 2011 Sep 15;184(6):627-9. doi: 10.1164/rccm.201107-1282ED. Am J Respir Crit Care Med. 2011. PMID: 21920923 No abstract available.
Similar articles
-
Transglutaminase 2: a novel therapeutic target for idiopathic pulmonary fibrosis using selective small molecule inhibitors.Amino Acids. 2021 Feb;53(2):205-217. doi: 10.1007/s00726-020-02938-w. Epub 2021 Jan 21. Amino Acids. 2021. PMID: 33474654 Free PMC article.
-
Spatially Resolved Identification of Transglutaminase Substrates by Proteomics in Pulmonary Fibrosis.Am J Respir Cell Mol Biol. 2021 Sep;65(3):319-330. doi: 10.1165/rcmb.2021-0012OC. Am J Respir Cell Mol Biol. 2021. PMID: 34264172
-
Transglutaminase 2 in pulmonary and cardiac tissue remodeling in experimental pulmonary hypertension.Am J Physiol Lung Cell Mol Physiol. 2017 Nov 1;313(5):L752-L762. doi: 10.1152/ajplung.00170.2017. Epub 2017 Aug 3. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 28775095 Free PMC article.
-
Implications of enigmatic transglutaminase 2 (TG2) in cardiac diseases and therapeutic developments.Biochem Pharmacol. 2022 Jul;201:115104. doi: 10.1016/j.bcp.2022.115104. Epub 2022 May 23. Biochem Pharmacol. 2022. PMID: 35617996 Review.
-
Role of Transglutaminase 2 in Cell Death, Survival, and Fibrosis.Cells. 2021 Jul 20;10(7):1842. doi: 10.3390/cells10071842. Cells. 2021. PMID: 34360011 Free PMC article. Review.
Cited by
-
Respiratory syncytial virus infection induces the release of transglutaminase 2 from human airway epithelial cells.Am J Physiol Lung Cell Mol Physiol. 2022 Jan 1;322(1):L1-L12. doi: 10.1152/ajplung.00013.2021. Epub 2021 Oct 27. Am J Physiol Lung Cell Mol Physiol. 2022. PMID: 34704843 Free PMC article.
-
Molecular programs of fibrotic change in aging human lung.Nat Commun. 2021 Nov 2;12(1):6309. doi: 10.1038/s41467-021-26603-2. Nat Commun. 2021. PMID: 34728633 Free PMC article.
-
Matrix biology of idiopathic pulmonary fibrosis: a workshop report of the national heart, lung, and blood institute.Am J Pathol. 2014 Jun;184(6):1643-51. doi: 10.1016/j.ajpath.2014.02.003. Epub 2014 Apr 13. Am J Pathol. 2014. PMID: 24726499 Free PMC article. Review.
-
Elevated transglutaminase-2 expression mediates fibrosis in areca quid chewing-associated oral submucocal fibrosis via reactive oxygen species generation.Clin Oral Investig. 2016 Jun;20(5):1029-34. doi: 10.1007/s00784-015-1579-0. Epub 2015 Sep 4. Clin Oral Investig. 2016. PMID: 26336810
-
Normal Human Lung Epithelial Cells Inhibit Transforming Growth Factor-β Induced Myofibroblast Differentiation via Prostaglandin E2.PLoS One. 2015 Aug 6;10(8):e0135266. doi: 10.1371/journal.pone.0135266. eCollection 2015. PLoS One. 2015. PMID: 26248335 Free PMC article.
References
-
- Swigris JJ, Kuschner WG, Kelsey JL, Gould MK. Idiopathic pulmonary fibrosis: challenges and opportunities for the clinician and investigator. Chest 2005;127:275–283 - PubMed
-
- Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2006;174:810–816 - PubMed
-
- Sime PJ, O'Reilly KM. Fibrosis of the lung and other tissues: new concepts in pathogenesis and treatment. Clin Immunol 2001;99:308–319 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 GM07356/GM/NIGMS NIH HHS/United States
- HL075432/HL/NHLBI NIH HHS/United States
- P30 ES001247/ES/NIEHS NIH HHS/United States
- T32 GM007356/GM/NIGMS NIH HHS/United States
- R03 HL095402/HL/NHLBI NIH HHS/United States
- F30 HL097596/HL/NHLBI NIH HHS/United States
- T32 HL66988/HL/NHLBI NIH HHS/United States
- R01 HL075432/HL/NHLBI NIH HHS/United States
- HL095402/HL/NHLBI NIH HHS/United States
- T32 ES007026/ES/NIEHS NIH HHS/United States
- T32 HL066988/HL/NHLBI NIH HHS/United States
- T32 ES07026/ES/NIEHS NIH HHS/United States
- HL075432-04S2/HL/NHLBI NIH HHS/United States
- 30 HL097596/HL/NHLBI NIH HHS/United States
- HL075432-04S1/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

