Integrin α6β4 identifies an adult distal lung epithelial population with regenerative potential in mice
- PMID: 21701069
- PMCID: PMC3223845
- DOI: 10.1172/JCI57673
Integrin α6β4 identifies an adult distal lung epithelial population with regenerative potential in mice
Erratum in
- J Clin Invest. 2011 Aug 1;121(8):3360
Abstract
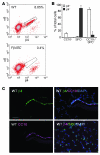
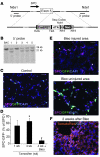
Laminins and their integrin receptors are implicated in epithelial cell differentiation and progenitor cell maintenance. We report here that a previously unrecognized subpopulation of mouse alveolar epithelial cells (AECs) expressing the laminin receptor α6β4, but little or no pro-surfactant C (pro-SPC), is endowed with regenerative potential. Ex vivo, this subpopulation expanded clonally as progenitors but also differentiated toward mature cell types. Integrin β4 itself was not required for AEC proliferation or differentiation. An in vivo embryonic lung organoid assay, which we believe to be novel, was used to show that purified β4+ adult AECs admixed with E14.5 lung single-cell suspensions and implanted under kidney capsules self-organized into distinct Clara cell 10-kDa secretory protein (CC10+) airway-like and SPC+ saccular structures within 6 days. Using a bleomycin model of lung injury and an SPC-driven inducible cre to fate-map AECs, we found the majority of type II AECs in fibrotic areas were not derived from preexisting type II AECs, demonstrating that SPC- progenitor cells replenished type II AECs during repair. Our findings support the idea that there is a stable AEC progenitor population in the adult lung, provide in vivo evidence of AEC progenitor cell differentiation after parenchymal injury, and identify a strong candidate progenitor cell for maintenance of type II AECs during lung repair.
Figures




Comment in
-
Integrin α6β4 defines a novel lung epithelial progenitor cell: a step forward for cell-based therapies for pulmonary disease.J Clin Invest. 2011 Jul;121(7):2543-5. doi: 10.1172/JCI58704. Epub 2011 Jun 23. J Clin Invest. 2011. PMID: 21701072 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

