Epidermal growth factor improves survival and prevents intestinal injury in a murine model of pseudomonas aeruginosa pneumonia
- PMID: 21701422
- PMCID: PMC3175258
- DOI: 10.1097/SHK.0b013e31822793c4
Epidermal growth factor improves survival and prevents intestinal injury in a murine model of pseudomonas aeruginosa pneumonia
Abstract
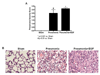
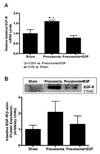
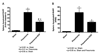
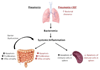
Mortality from pneumonia is mediated, in part, through extrapulmonary causes. Epidermal growth factor (EGF) has broad cytoprotective effects, including potent restorative properties in the injured intestine. The purpose of this study was to determine the efficacy of EGF treatment following Pseudomonas aeruginosa pneumonia. FVB/N mice underwent intratracheal injection of either P. aeruginosa or saline and were then randomized to receive either systemic EGF or vehicle beginning immediately or 24 h after the onset of pneumonia. Systemic EGF decreased 7-day mortality from 65% to 10% when initiated immediately after the onset of pneumonia and to 27% when initiated 24 h after the onset of pneumonia. Even though injury in pneumonia is initiated in the lungs, the survival advantage conferred by EGF was not associated with improvements in pulmonary pathology. In contrast, EGF prevented intestinal injury by reversing pneumonia-induced increases in intestinal epithelial apoptosis and decreases in intestinal proliferation and villus length. Systemic cytokines and kidney and liver function were unaffected by EGF therapy, although EGF decreased pneumonia-induced splenocyte apoptosis. To determine whether the intestine was sufficient to account for extrapulmonary effects induced by EGF, a separate set of experiments was done using transgenic mice with enterocyte-specific overexpression of EGF (IFABP-EGF [intestinal fatty acid-binding protein linked to mouse EGF] mice), which were compared with wild-type mice subjected to pneumonia. IFABP-EGF mice had improved survival compared with wild-type mice following pneumonia (50% vs. 28%, respectively, P < 0.05) and were protected from pneumonia-induced intestinal injury. Thus, EGF may be a potential adjunctive therapy for pneumonia, mediated in part by its effects on the intestine.
Figures




References
-
- Heron M, Hoyert DL, Murphy SL, Xu J, Kochanek KD, Tejada-Vera B. Deaths: final data for 2006. Natl Vital Stat Rep 17. 2009;57(14):1–134. - PubMed
-
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20(6):864–874. - PubMed
-
- Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Post-injury multiple organ failure: the role of the gut. Shock. 2001;15(1):1–10. - PubMed
-
- Coopersmith CM, Stromberg PE, Dunne WM, Davis CG, Amiot DM, Buchman TG, Karl IE, Hotchkiss RS. Inhibition of intestinal epithelial apoptosis and survival in a murine model of pneumonia-induced sepsis. JAMA. 2002;287(13):1716–1721. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

