p53 independent epigenetic-differentiation treatment in xenotransplant models of acute myeloid leukemia
- PMID: 21701495
- PMCID: PMC3668642
- DOI: 10.1038/leu.2011.159
p53 independent epigenetic-differentiation treatment in xenotransplant models of acute myeloid leukemia
Abstract
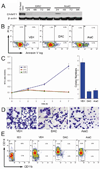
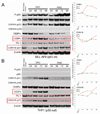
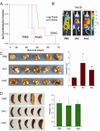
Suppression of apoptosis by TP53 mutation contributes to resistance of acute myeloid leukemia (AML) to conventional cytotoxic treatment. Using differentiation to induce irreversible cell cycle exit in AML cells could be a p53-independent treatment alternative, however, this possibility requires evaluation. In vitro and in vivo regimens of the deoxycytidine analogue decitabine that deplete the chromatin-modifying enzyme DNA methyl-transferase 1 without phosphorylating p53 or inducing early apoptosis were determined. These decitabine regimens but not equimolar DNA-damaging cytarabine upregulated the key late differentiation factors CCAAT enhancer-binding protein ɛ and p27/cyclin dependent kinase inhibitor 1B (CDKN1B), induced cellular differentiation and terminated AML cell cycle, even in cytarabine-resistant p53- and p16/CDKN2A-null AML cells. Leukemia initiation by xenotransplanted AML cells was abrogated but normal hematopoietic stem cell engraftment was preserved. In vivo, the low toxicity allowed frequent drug administration to increase exposure, an important consideration for S phase specific decitabine therapy. In xenotransplant models of p53-null and relapsed/refractory AML, the non-cytotoxic regimen significantly extended survival compared with conventional cytotoxic cytarabine. Modifying in vivo dose and schedule to emphasize this pathway of decitabine action can bypass a mechanism of resistance to standard therapy.
Figures



References
-
- Vazquez A, Bond EE, Levine AJ, Bond GL. The genetics of the p53 pathway, apoptosis and cancer therapy. Nat Rev Drug Discov. 2008;7(12):979–987. - PubMed
-
- Suarez L, Vidriales MB, Garcia-Larana J, Sanz G, Moreno MJ, Lopez A, Barrena S, Martinez R, Tormo M, Palomera L, Lavilla E, Lopez-Berges MC, de Santiago M, de Equiza ME, Miguel JF, Orfao A. CD34+ cells from acute myeloid leukemia, myelodysplastic syndromes, and normal bone marrow display different apoptosis and drug resistance-associated phenotypes. Clin Cancer Res. 2004;10(22):7599–7606. - PubMed
-
- Yin B, Kogan SC, Dickins RA, Lowe SW, Largaespada DA. Trp53 loss during in vitro selection contributes to acquired Ara-C resistance in acute myeloid leukemia. Exp Hematol. 2006;34(5):631–641. - PubMed
-
- Wattel E, Preudhomme C, Hecquet B, Vanrumbeke M, Quesnel B, Dervite I, Morel P, Fenaux P. p53 mutations are associated with resistance to chemotherapy and short survival in hematologic malignancies. Blood. 1994;84(9):3148–3157. - PubMed
-
- Toledo F, Wahl GM. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat Rev Cancer. 2006;6(12):909–923. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

