Signal integration and crosstalk during thymocyte migration and emigration
- PMID: 21701522
- PMCID: PMC3710714
- DOI: 10.1038/nri2989
Signal integration and crosstalk during thymocyte migration and emigration
Abstract
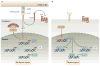
The thymus produces self-tolerant functionally competent T cells. This process involves the import of multipotent haematopoietic progenitors that are then signalled to adopt the T cell fate. Expression of T cell-specific genes, including those encoding the T cell receptor (TCR), is followed by positive and negative selection and the eventual export of mature T cells. Significant progress has been made in elucidating the signals that direct progenitor cell trafficking to, within and out of the thymus. These advances are the subject of this Review, with a particular focus on the role of reciprocal cooperative and regulatory interactions between TCR- and chemokine receptor-mediated signalling.
Figures

References
-
- Bhandoola A, von Boehmer H, Petrie HT, Zuniga-Pflucker JC. Commitment and developmental potential of extrathymic and intrathymic T cell precursors: plenty to choose from. Immunity. 2007;26:678–89. - PubMed
-
- Petrie HT, Zuniga-Pflucker JC. Zoned out: functional mapping of stromal signaling microenvironments in the thymus. Annu Rev Immunol. 2007;25:649–79. - PubMed
-
- Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol. 2006;6:127–35. - PubMed
-
- Hollander G, et al. Cellular and molecular events during early thymus development. Immunol Rev. 2006;209:28–46. - PubMed
-
- Boehm T. Thymus development and function. Curr Opin Immunol. 2008;20:178–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 HL086900/HL/NHLBI NIH HHS/United States
- Z99 OD999999/ImNIH/Intramural NIH HHS/United States
- Z99 MH999999/ImNIH/Intramural NIH HHS/United States
- R01 AI059621/AI/NIAID NIH HHS/United States
- Z99 HD999999/ImNIH/Intramural NIH HHS/United States
- ZIA HD001803/ImNIH/Intramural NIH HHS/United States
- RC1 HL099758/HL/NHLBI NIH HHS/United States
- RC1HL099758/HL/NHLBI NIH HHS/United States
- R01 HL110741/HL/NHLBI NIH HHS/United States
- Z99 DA999999/ImNIH/Intramural NIH HHS/United States
- AI059621/AI/NIAID NIH HHS/United States
- Z99 HL999999/ImNIH/Intramural NIH HHS/United States
LinkOut - more resources
Full Text Sources

