DNA methylation status predicts cell type-specific enhancer activity
- PMID: 21701563
- PMCID: PMC3160184
- DOI: 10.1038/emboj.2011.210
DNA methylation status predicts cell type-specific enhancer activity
Abstract
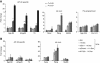
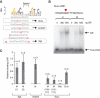
Cell-selective glucocorticoid receptor (GR) binding to distal regulatory elements is associated with cell type-specific regions of locally accessible chromatin. These regions can either pre-exist in chromatin (pre-programmed) or be induced by the receptor (de novo). Mechanisms that create and maintain these sites are not well understood. We observe a global enrichment of CpG density for pre-programmed elements, and implicate their demethylated state in the maintenance of open chromatin in a tissue-specific manner. In contrast, sites that are actively opened by GR (de novo) are characterized by low CpG density, and form a unique class of enhancers devoid of suppressive effect of agglomerated methyl-cytosines. Furthermore, treatment with glucocorticoids induces rapid changes in methylation levels at selected CpGs within de novo sites. Finally, we identify GR-binding elements with CpGs at critical positions, and show that methylation can affect GR-DNA interactions in vitro. The findings present a unique link between tissue-specific chromatin accessibility, DNA methylation and transcription factor binding and show that DNA methylation can be an integral component of gene regulation by nuclear receptors.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Angrisano T, Sacchetti S, Natale F, Cerrato A, Pero R, Keller S, Peluso S, Perillo B, Avvedimento VE, Fusco A, Bruni CB, Lembo F, Santoro M, Chiariotti L (2010) Chromatin and DNA methylation dynamics during retinoic acid-induced RET gene transcriptional activation in neuroblastoma cells. Nucleic Acids Res 38: 1993–2006 - PMC - PubMed
-
- Antequera F, Macleod D, Bird AP (1989) Specific protection of methylated CpGs in mammalian nuclei. Cell 58: 509–517 - PubMed
-
- Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K (2007) High-resolution profiling of histone methylations in the human genome. Cell 129: 823–837 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

