Prevention of NSAID-related upper gastrointestinal toxicity: a meta-analysis of traditional NSAIDs with gastroprotection and COX-2 inhibitors
- PMID: 21701610
- PMCID: PMC3108684
- DOI: 10.2147/dhps.s4334
Prevention of NSAID-related upper gastrointestinal toxicity: a meta-analysis of traditional NSAIDs with gastroprotection and COX-2 inhibitors
Abstract
Background: Traditional NSAIDs (tNSAIDs) and COX-2 inhibitors (COX-2s) are important agents for the treatment of a variety or arthritic conditions. The purpose of this study was to systematically review the effectiveness of misoprostol, H2-receptor antagonists (H2RAs), and proton pump inhibitors (PPIs) for the prevention of tNSAID related upper gastrointestinal (GI) toxicity, and to review the upper gastrointestinal (GI) safety of COX-2s.
Methods: An extensive literature search was performed to identify randomized controlled trials (RCTs) of prophylactic agents used for the prevention of upper GI toxicity, and RCTs that assessed the GI safety of the newer COX-2s. Meta-analysis was performed in accordance with accepted techniques.
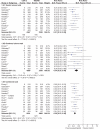
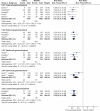
Results: 39 gastroprotection and 69 COX-2 RCTs met inclusion criteria. Misoprostol, PPIs, and double doses of H2RAs are effective at reducing the risk of both endoscopic gastric and duodenal tNSAID-induced ulcers. Standard doses of H2RAs are not effective at reducing the risk of tNSAID-induced gastric ulcers, but reduce the risk of duodenal ulcers. Misoprostol is associated with greater adverse effects than the other agents, particularly at higher doses. COX-2s are associated with fewer endoscopic ulcers and clinically important ulcer complications, and have fewer treatment withdrawals due to GI symptoms than tNSAIDS. Acetylsalicylic acid appears to diminish the benefit of COX-2s over tNSAIDs. In high risk GI patients, tNSAID with a PPI or a COX-2 alone appear to offer similar GI safety, but a strategy of a COX-2 with a PPI appears to offer the greatest GI safety.
Conclusion: Several strategies are available to reduce the risk of upper GI toxicity with tNSAIDs. The choice between these strategies needs to consider patients' underlying GI and cardiovascular risk.
Keywords: COX-2 inhibitors; NSAID; gastrointestinal toxicity.
Figures




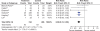
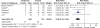
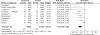

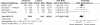
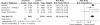
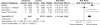
References
-
- Barnard L, Lavoie D, Lajeunesse N. Increase in nonfatal digestive perforations and haemorrhages following introduction of selective NSAIDs: a public health concern. Drug Saf. 2006;29(7):613–620. - PubMed
-
- Garcia RL, Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugs. Lancet. 1994;343(8900):769–772. - PubMed
-
- Rostom A, Wells G, Tugwell P, Welch V, Dube C, McGowan J. The prevention of chronic NSAID induced upper gastrointestinal toxicity: a Cochrane collaboration metaanalysis of randomized controlled trials. J Rheumatol. 2000;27(9):2203–2214. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

