Humoral response to catumaxomab correlates with clinical outcome: results of the pivotal phase II/III study in patients with malignant ascites
- PMID: 21702044
- PMCID: PMC3415680
- DOI: 10.1002/ijc.26258
Humoral response to catumaxomab correlates with clinical outcome: results of the pivotal phase II/III study in patients with malignant ascites
Abstract
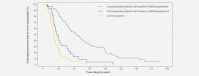
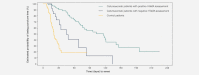
The trifunctional antibody catumaxomab is a targeted immunotherapy for the intraperitoneal treatment of malignant ascites. In a Phase II/III trial in cancer patients (n = 258) with malignant ascites, catumaxomab showed a clear clinical benefit vs. paracentesis and had an acceptable safety profile. Human antimouse antibodies (HAMAs), which could be associated with beneficial humoral effects and prolonged survival, may develop against catumaxomab as it is a mouse/rat antibody. This post hoc analysis investigated whether there was a correlation between the detection of HAMAs 8 days after the fourth catumaxomab infusion and clinical outcome. HAMA-positive and HAMA-negative patients in the catumaxomab group and patients in the control group were analyzed separately for all three clinical outcome measures (puncture-free survival, time to next puncture and overall survival) and compared to each other. There was a strong correlation between humoral response and clinical outcome: patients who developed HAMAs after catumaxomab showed significant improvement in all three clinical outcome measures vs. HAMA-negative patients. In the overall population in HAMA-positive vs. HAMA-negative patients, median puncture-free survival was 64 vs. 27 days (p < 0.0001; HR 0.330), median time to next therapeutic puncture was 104 vs. 46 days (p = 0.0002; HR 0.307) and median overall survival was 129 vs. 64 days (p = 0.0003; HR 0.433). Similar differences between HAMA-positive and HAMA-negative patients were seen in the ovarian, nonovarian and gastric cancer subgroups. In conclusion, HAMA development may be a biomarker for catumaxomab response and patients who developed HAMAs sooner derived greater benefit from catumaxomab treatment.
Copyright © 2011 UICC.
Figures
References
-
- Zeidler R, Reisbach G, Wollenberg B, Lang S, Chaubal S, Schmitt B, Lindhofer H. Simultaneous activation of T-cells and accessory cells by a new class of intact bispecific antibody results in efficient tumour cell killing. J Immunol. 1999;163:1246–52. - PubMed
-
- Seimetz D, Lindhofer H, Bokemeyer C. Development and approval of the trifunctional antibody catumaxomab (anti-EpCAMxanti-CD3) as a targeted cancer immunotherapy. Cancer Treat Rev. 2010;36:458–67. - PubMed
-
- Ruf P, Lindhofer H. Induction of a long-lasting antitumor immunity by a trifunctional bispecific antibody. Blood. 2001;98:2526–34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical