Delivery of cefotaxime to the brain via intranasal administration
- PMID: 21702731
- PMCID: PMC5598079
- DOI: 10.3109/03639045.2011.571696
Delivery of cefotaxime to the brain via intranasal administration
Abstract
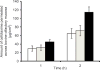
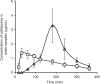
The purpose of this study was to investigate the plausibility of delivery of cefotaxime to the brain via intranasal administration. In vitro permeation studies were carried out using Franz diffusion cells, and the effect of different concentrations of chitosan (0.1% w/v and 0.25% w/v) on drug permeation across the bovine olfactory mucosa was determined. Samples were collected from the receiver compartment at different time points and analyzed using HPLC. The amount of cefotaxime that permeated across the olfactory mucosa when 0.25% w/v of chitosan was used as a permeation enhancer was ~1.5- and ~2-fold higher at the end of the first hour and second hour, respectively, over control (29.56 ± 6.18 µg/cm(2)). There was no significant enhancement in drug permeation when 0.1% w/v chitosan was used as the permeation enhancer. Pharmacokinetic studies were carried out using Sprague-Dawley rats. Cefotaxime solution with 0.25% w/v chitosan (40 mg/kg) was administered intravenously (i.v.) to rats in groups 1 and 3 and intranasally to those in group 2 and 4. The time course of drug in the brain was investigated by performing microdialysis in rats of groups 1 and 2. Blood samples were withdrawn from rats in groups 3 and 4, and cefotaxime in plasma was analyzed using HPLC after extraction with a hydrochloric acid-chloroform:1-pentanol (3:1) and phosphate buffer solvent system. Pharmacokinetic parameters were calculated using the trapezoidal rule. The results imply that the drug levels attained in the brain following i.v. and intranasal administrations were comparable. These results suggest that intranasal administration of cefotaxime could be a potential method of delivering antibacterial agents because of it being noninvasive and patient compliant.
Conflict of interest statement
The authors report no conflicts of interest.
Figures

References
-
- Nelson JD. Emerging role of cephalosporins in bacterial meningitis. Am J Med. 1985;79:47–51. - PubMed
-
- Puddicombe JB, Wali SS, Greenwood BM. A field trial of a single intramuscular injection of long-acting chloramphenicol in the treatment of meningococcal meningitis. Trans R Soc Trop Med Hyg. 1984;78:399–403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
