Metagenomic biomarker discovery and explanation
- PMID: 21702898
- PMCID: PMC3218848
- DOI: 10.1186/gb-2011-12-6-r60
Metagenomic biomarker discovery and explanation
Abstract
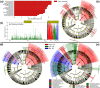
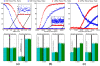
This study describes and validates a new method for metagenomic biomarker discovery by way of class comparison, tests of biological consistency and effect size estimation. This addresses the challenge of finding organisms, genes, or pathways that consistently explain the differences between two or more microbial communities, which is a central problem to the study of metagenomics. We extensively validate our method on several microbiomes and a convenient online interface for the method is provided at http://huttenhower.sph.harvard.edu/lefse/.
Figures




References
-
- Tothill RW, Tinker AV, George J, Brown R, Fox SB, Lade S, Johnson DS, Trivett MK, Etemadmoghadam D, Locandro B, Traficante N, Fereday S, Hung JA, Chiew YE, Haviv I. Australian Ovarian Cancer Study Group; Gertig D, DeFazio A, Bowtell DD. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res. 2008;14:5198–5208. doi: 10.1158/1078-0432.CCR-08-0196. - DOI - PubMed
-
- De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. 2010;107:14691–14696. doi: 10.1073/pnas.1005963107. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

