Accounting for the mortality benefit of drug-eluting stents in percutaneous coronary intervention: a comparison of methods in a retrospective cohort study
- PMID: 21702899
- PMCID: PMC3141543
- DOI: 10.1186/1741-7015-9-78
Accounting for the mortality benefit of drug-eluting stents in percutaneous coronary intervention: a comparison of methods in a retrospective cohort study
Abstract
Background: Drug-eluting stents (DES) reduce rates of restenosis compared with bare metal stents (BMS). A number of observational studies have also found lower rates of mortality and non-fatal myocardial infarction with DES compared with BMS, findings not observed in randomized clinical trials. In order to explore reasons for this discrepancy, we compared outcomes after percutaneous coronary intervention (PCI) with DES or BMS by multiple statistical methods.
Methods: We compared short-term rates of all-cause mortality and myocardial infarction for patients undergoing PCI with DES or BMS using propensity-score adjustment, propensity-score matching, and a stent-era comparison in a large, integrated health system between 1998 and 2007. For the propensity-score adjustment and stent era comparisons, we used multivariable logistic regression to assess the association of stent type with outcomes. We used McNemar's Chi-square test to compare outcomes for propensity-score matching.
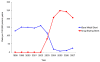
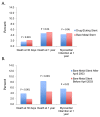
Results: Between 1998 and 2007, 35,438 PCIs with stenting were performed among health plan members (53.9% DES and 46.1% BMS). After propensity-score adjustment, DES was associated with significantly lower rates of death at 30 days (OR 0.49, 95% CI 0.39 - 0.63, P < 0.001) and one year (OR 0.58, 95% CI 0.49 - 0.68, P < 0.001), and a lower rate of myocardial infarction at one year (OR 0.72, 95% CI 0.59 - 0.87, P < 0.001). Thirty day and one year mortality were also lower with DES after propensity-score matching. However, a stent era comparison, which eliminates potential confounding by indication, showed no difference in death or myocardial infarction for DES and BMS, similar to results from randomized trials.
Conclusions: Although propensity-score methods suggested a mortality benefit with DES, consistent with prior observational studies, a stent era comparison failed to support this conclusion. Unobserved factors influencing stent selection in observational studies likely account for the observed mortality benefit of DES not seen in randomized clinical trials.
Figures

References
-
- The American Recovery and Reinvestment Act of 2009. H.R.1.ENR. http://www.recovery.gov/About/Pages/The_Act.aspx (Accessed July 4, 2011)
-
- Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–763. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

