Hydroxylation of recombinant human collagen type I alpha 1 in transgenic maize co-expressed with a recombinant human prolyl 4-hydroxylase
- PMID: 21702901
- PMCID: PMC3151215
- DOI: 10.1186/1472-6750-11-69
Hydroxylation of recombinant human collagen type I alpha 1 in transgenic maize co-expressed with a recombinant human prolyl 4-hydroxylase
Abstract
Background: Collagens require the hydroxylation of proline (Pro) residues in their triple-helical domain repeating sequence Xaa-Pro-Gly to function properly as a main structural component of the extracellular matrix in animals at physiologically relevant conditions. The regioselective proline hydroxylation is catalyzed by a specific prolyl 4-hydroxylase (P4H) as a posttranslational processing step.
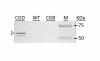
Results: A recombinant human collagen type I α-1 (rCIα1) with high percentage of hydroxylated prolines (Hyp) was produced in transgenic maize seeds when co-expressed with both the α- and β- subunits of a recombinant human P4H (rP4H). Germ-specific expression of rCIα1 using maize globulin-1 gene promoter resulted in an average yield of 12 mg/kg seed for the full-length rCIα1 in seeds without co-expression of rP4H and 4 mg/kg seed for the rCIα1 (rCIα1-OH) in seeds with co-expression of rP4H. High-resolution mass spectrometry (HRMS) analysis revealed that nearly half of the collagenous repeating triplets in rCIα1 isolated from rP4H co-expressing maize line had the Pro residues changed to Hyp residues. The HRMS analysis determined the Hyp content of maize-derived rCIα1-OH as 18.11%, which is comparable to the Hyp level of yeast-derived rCIα1-OH (17.47%) and the native human CIa1 (14.59%), respectively. The increased Hyp percentage was correlated with a markedly enhanced thermal stability of maize-derived rCIα1-OH when compared to the non-hydroxylated rCIα1.
Conclusions: This work shows that maize has potential to produce adequately modified exogenous proteins with mammalian-like post-translational modifications that may be require for their use as pharmaceutical and industrial products.
Figures
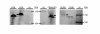

Similar articles
-
Purification and characterization of a transgenic corn grain-derived recombinant collagen type I alpha 1.Biotechnol Prog. 2009 Nov-Dec;25(6):1660-8. doi: 10.1002/btpr.257. Biotechnol Prog. 2009. PMID: 19637392
-
Collagen prolyl 4-hydroxylase isoenzymes I and II have sequence specificity towards different X-Pro-Gly triplets.Matrix Biol. 2024 Jan;125:73-87. doi: 10.1016/j.matbio.2023.12.001. Epub 2023 Dec 9. Matrix Biol. 2024. PMID: 38081527
-
Characterization of a second Arabidopsis thaliana prolyl 4-hydroxylase with distinct substrate specificity.J Biol Chem. 2005 Jan 14;280(2):1142-8. doi: 10.1074/jbc.M411109200. Epub 2004 Nov 4. J Biol Chem. 2005. PMID: 15528200
-
Role of prolyl hydroxylation in the molecular interactions of collagens.Essays Biochem. 2019 Sep 13;63(3):325-335. doi: 10.1042/EBC20180053. Print 2019 Sep 13. Essays Biochem. 2019. PMID: 31350381 Free PMC article. Review.
-
Prolyl 4-hydroxylase.Crit Rev Biochem Mol Biol. 2010 Apr;45(2):106-24. doi: 10.3109/10409231003627991. Crit Rev Biochem Mol Biol. 2010. PMID: 20199358 Free PMC article. Review.
Cited by
-
Hybrid Freeze-Dried Dressings Composed of Epidermal Growth Factor and Recombinant Human-Like Collagen Enhance Cutaneous Wound Healing in Rats.Front Bioeng Biotechnol. 2020 Jul 15;8:742. doi: 10.3389/fbioe.2020.00742. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32760705 Free PMC article.
-
Collagen Type II-Chitosan Interactions as Dependent on Hydroxylation and Acetylation Inferred from Molecular Dynamics Simulations.Molecules. 2022 Dec 24;28(1):154. doi: 10.3390/molecules28010154. Molecules. 2022. PMID: 36615345 Free PMC article.
-
Engineered recombinant bacterial collagen as an alternative collagen-based biomaterial for tissue engineering.Front Chem. 2014 Jun 23;2:40. doi: 10.3389/fchem.2014.00040. eCollection 2014. Front Chem. 2014. PMID: 25003103 Free PMC article. No abstract available.
-
Innovations and challenges in collagen and gelatin production through precision fermentation.World J Microbiol Biotechnol. 2025 Feb 6;41(2):63. doi: 10.1007/s11274-025-04276-z. World J Microbiol Biotechnol. 2025. PMID: 39910024 Review.
-
Production of a functional human acid maltase in tobacco seeds: biochemical analysis, uptake by human GSDII cells, and in vivo studies in GAA knockout mice.Appl Biochem Biotechnol. 2013 Oct;171(4):916-26. doi: 10.1007/s12010-013-0367-z. Epub 2013 Aug 2. Appl Biochem Biotechnol. 2013. PMID: 23907679 Free PMC article.
References
-
- Vanderrest M, Garrone R. Collagen Family of Proteins. FASEB Journal. 1991;5:2814–2823. - PubMed
-
- Geddis AE, Prockop DJ. Expression of human COL1A1 gene in stably transfected HT1080 cells: the production of a thermostable homotrimer of type I collagen in a recombinant system. Matrix. 1993;13:399–405. - PubMed
-
- Myllyharju J, Lamberg A, Notbohm H, Fietzek PP, Pihlajaniemi T, Kivirikko KI. Expression of wild-type and modified pro alpha chains of human type I procollagen in insect cells leads to the formation of stable [alpha 1(I)](2)alpha 2(I) collagen heterotrimers and [alpha 1(I)](3) homotrimers but not [alpha 2(I)](3) homotrimers. Journal of Biological Chemistry. 1997;272:21824–21830. doi: 10.1074/jbc.272.35.21824. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

