Novel multiplex technology for diagnostic characterization of rheumatoid arthritis
- PMID: 21702928
- PMCID: PMC3218917
- DOI: 10.1186/ar3383
Novel multiplex technology for diagnostic characterization of rheumatoid arthritis
Abstract
Introduction: The aim of this study was to develop a clinical-grade, automated, multiplex system for the differential diagnosis and molecular stratification of rheumatoid arthritis (RA).
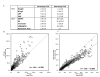
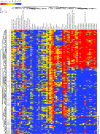
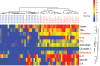
Methods: We profiled autoantibodies, cytokines, and bone-turnover products in sera from 120 patients with a diagnosis of RA of < 6 months' duration, as well as in sera from 27 patients with ankylosing spondylitis, 28 patients with psoriatic arthritis, and 25 healthy individuals. We used a commercial bead assay to measure cytokine levels and developed an array assay based on novel multiplex technology (Immunological Multi-Parameter Chip Technology) to evaluate autoantibody reactivities and bone-turnover markers. Data were analyzed by Significance Analysis of Microarrays and hierarchical clustering software.
Results: We developed a highly reproducible, automated, multiplex biomarker assay that can reliably distinguish between RA patients and healthy individuals or patients with other inflammatory arthritides. Identification of distinct biomarker signatures enabled molecular stratification of early-stage RA into clinically relevant subtypes. In this initial study, multiplex measurement of a subset of the differentiating biomarkers provided high sensitivity and specificity in the diagnostic discrimination of RA: Use of 3 biomarkers yielded a sensitivity of 84.2% and a specificity of 93.8%, and use of 4 biomarkers a sensitivity of 59.2% and a specificity of 96.3%.
Conclusions: The multiplex biomarker assay described herein has the potential to diagnose RA with greater sensitivity and specificity than do current clinical tests. Its ability to stratify RA patients in an automated and reproducible manner paves the way for the development of assays that can guide RA therapy.
Figures




Comment in
-
Multiplex, megaplex, index, and complex: the present and future of laboratory diagnostics in rheumatology.Arthritis Res Ther. 2011;13(6):134. doi: 10.1186/ar3498. Epub 2011 Nov 24. Arthritis Res Ther. 2011. PMID: 22129036 Free PMC article.
References
-
- Hueber W, Tomooka BH, Zhao X, Kidd BA, Drijfhout JW, Fries JF, van Venrooij WJ, Metzger AL, Genovese MC, Robinson WH. Proteomic analysis of secreted proteins in early rheumatoid arthritis: anti-citrulline autoreactivity is associated with up regulation of proinflammatory cytokines. Ann Rheum Dis. 2007;66:712–719. doi: 10.1136/ard.2006.054924. - DOI - PMC - PubMed
-
- Kievit W, Fransen J, Oerlemans AJ, Kuper HH, van der Laar MA, de Rooij DJ, De Gendt CM, Ronday KH, Jansen TL, van Oijen PC, Brus HL, Adang EM, van Riel PL. The efficacy of anti-TNF in rheumatoid arthritis, a comparison between randomised controlled trials and clinical practice. Ann Rheum Dis. 2007;66:1473–1478. doi: 10.1136/ard.2007.072447. - DOI - PMC - PubMed
-
- Hueber W, Tomooka BH, Batliwalla F, Li W, Monach PA, Tibshirani RJ, Van Vollenhoven RF, Lampa J, Saito K, Tanaka Y, Genovese MC, Klareskog L, Gregersen PK, Robinson WH. Blood autoantibody and cytokine profiles predict response to anti-tumor necrosis factor therapy in rheumatoid arthritis. Arthritis Res Ther. 2009;11:R76. doi: 10.1186/ar2706. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

