Dietary flaxseed administered post thoracic radiation treatment improves survival and mitigates radiation-induced pneumonopathy in mice
- PMID: 21702963
- PMCID: PMC3146937
- DOI: 10.1186/1471-2407-11-269
Dietary flaxseed administered post thoracic radiation treatment improves survival and mitigates radiation-induced pneumonopathy in mice
Abstract
Background: Flaxseed (FS) is a dietary supplement known for its antioxidant and anti-inflammatory properties. Radiation exposure of lung tissues occurs either when given therapeutically to treat intrathoracic malignancies or incidentally, such as in the case of exposure from inhaled radioisotopes released after the detonation of a radiological dispersion devise (RDD). Such exposure is associated with pulmonary inflammation, oxidative tissue damage and irreversible lung fibrosis. We previously reported that dietary FS prevents pneumonopathy in a rodent model of thoracic X-ray radiation therapy (XRT). However, flaxseed's therapeutic usefulness in mitigating radiation effects post-exposure has never been evaluated.
Methods: We evaluated the effects of a 10%FS or isocaloric control diet given to mice (C57/BL6) in 2 separate experiments (n = 15-25 mice/group) on 0, 2, 4, 6 weeks post a single dose 13.5 Gy thoracic XRT and compared it to an established radiation-protective diet given preventively, starting at 3 weeks prior to XRT. Lungs were evaluated four months post-XRT for blood oxygenation levels, inflammation and fibrosis.
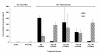
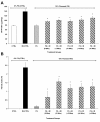
Results: Irradiated mice fed a 0%FS diet had a 4-month survival rate of 40% as compared to 70-88% survival in irradiated FS-fed mouse groups. Additionally, all irradiated FS-fed mice had decreased fibrosis compared to those fed 0%FS. Lung OH-Proline content ranged from 96.5 ± 7.1 to 110.2 ± 7.7 μg/ml (Mean ± SEM) in all irradiated FS-fed mouse groups, as compared to 138 ± 10.8 μg/ml for mice on 0%FS. Concomitantly, bronchoalveolar lavage (BAL) protein and weight loss associated with radiation cachexia was significantly decreased in all FS-fed groups. Inflammatory cell influx to lungs also decreased significantly except when FS diet was delayed by 4 and 6 weeks post XRT. All FS-fed mice (irradiated or not), maintained a higher blood oxygenation level as compared to mice on 0%FS. Similarly, multiplex cytokine analysis in the BAL fluid revealed a significant decrease of specific inflammatory cytokines in FS-fed mice.
Conclusions: Dietary FS given post-XRT mitigates radiation effects by decreasing pulmonary fibrosis, inflammation, cytokine secretion and lung damage while enhancing mouse survival. Dietary supplementation of FS may be a useful adjuvant treatment mitigating adverse effects of radiation in individuals exposed to inhaled radioisotopes or incidental radiation.
Figures
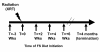






References
-
- Chin FK. Scenario of a dirty bomb in an urban environment and acute management of radiation poisoning and injuries. Singapore Med J. 2007;48(10):950–957. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

