Protein kinase CK2α is overexpressed in colorectal cancer and modulates cell proliferation and invasion via regulating EMT-related genes
- PMID: 21702981
- PMCID: PMC3132712
- DOI: 10.1186/1479-5876-9-97
Protein kinase CK2α is overexpressed in colorectal cancer and modulates cell proliferation and invasion via regulating EMT-related genes
Abstract
Background: Protein kinase CK2 is a highly conserved, ubiquitous protein serine/threonine kinase that phosphorylates many substrates and has a global role in numerous biological and pathological processes. Overexpression of the protein kinase CK2α subunit (CK2α) has been associated with the malignant transformation of several tissues, with not nearly as much focus on the role of CK2α in colorectal cancer (CRC). The aims of this study are to investigate the function and regulatory mechanism of CK2α in CRC development.
Methods: Expression levels of CK2α were analyzed in 144 patients (104 with CRC and 40 with colorectal adenoma) by immunohistochemistry. Proliferation, senescence, motility and invasion assays as well as immunofluorescence staining and western blots were performed to assess the effect of CK2α in CRC.
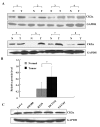
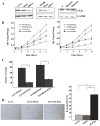
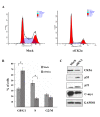
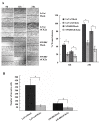
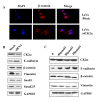
Results: The immunohistochemical expression of nuclear CK2α was stronger in tumor tissues than in adenomas and normal colorectal tissues. Suppression of CK2α by small-interfering RNA or the CK2α activity inhibitor emodin inhibited proliferation of CRC cells, caused G0/G1 phase arrest, induced cell senescence, elevated the expression of p53/p21 and decreased the expression of C-myc. We also found that knockdown of CK2α suppressed cell motility and invasion. Significantly, CK2α inhibition resulted in β-catenin transactivation, decreased the expression levels of vimentin and the transcription factors snail1 and smad2/3, and increased the expression of E-cadherin, suggesting that CK2α regulates the epithelial-mesenchymal transition (EMT) process in cancer cells.
Conclusions: Our results indicate that CK2α plays an essential role in the development of CRC, and inhibition of CK2α may serve as a promising therapeutic strategy for human CRC.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

