Cost of non-persistence with oral bisphosphonates in post-menopausal osteoporosis treatment in France
- PMID: 21702989
- PMCID: PMC3141385
- DOI: 10.1186/1472-6963-11-151
Cost of non-persistence with oral bisphosphonates in post-menopausal osteoporosis treatment in France
Abstract
Background: During the last decade, oral bisphosphonates (BP) became the most widely prescribed pharmacologic class for post-menopausal osteoporosis. However, many surveys revealed the important issue of poor persistence with those drugs resulting in a failure of treatment to reduce fracture risk sufficiently. Using a published Markov model, this study analyses the economic impact of non-persistence with bisphosphonates in the context of the introduction of generics in France.
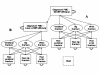
Methods: Direct costs of vertebral, hip and wrist fracture were assessed and included in an existing 10-year Markov model developed to analyse consequences of non-persistence. Three alternatives of comparison were set: no treatment, real-world persistence, and ideal persistence. Simulated patients' characteristics matched those from a French observational study and the real-world adherence alternative employed persistence data from published database analysis. The risk of fracture of menopausal women and the risk reduction associated with the drugs were based on results reported in clinical trials. Incremental cost-effectiveness ratios (ICERs) were calculated first between real-world adherence and no treatment alternatives, and second between ideal and real-world persistence alternatives. The cost of non-persistence was defined as the difference between total cost of ideal and real-world persistence alternatives.
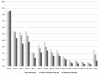
Results: Within fractured women population, mean costs of 10-year management of fracture were significantly different between the three alternatives with €7,239 (± €4,783), €6,711 (± €4,410) and €6,134 (± €3,945) in the no-treatment, the real-world and ideal persistence alternatives, respectively (p < 0.0001). Cost-effectiveness ratio for real-world treatment persistence compared with no-treatment alternative was found dominant and as well, alternative of ideal persistence dominated the former. Each ten percentage point of persistence gain amounted to €58 per patient, and extrapolation resulted in a global annual cost of non-persistence of over €30 million to the French health care system, with a substantial transfer from hospital to pharmacy budgets.
Conclusion: Within term, improving persistence with oral bisphosphonates should be economically dominant on levels currently known in real-world. Given this potential savings, ambitious adherence-enhancing interventions should be considered in osteoporotic patients.
Figures
References
-
- World Health Organization. Study Group Report: Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. World Health Organ Tech Rep Ser. 1994;843:1–129. - PubMed
-
- Ministry of Health and Solidarity (Ministère de la Santé et des Solidarités) Indicateurs de suivi de l'atteinte des 100 objectifs du rapport annexé à la loi du 9 août 2004 relative à la politique de santé publique [in French] La documentation française. 2005. http://www.ladocumentationfrancaise.fr/rapports/index.shtml
-
- Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs RW Jr, Dequeker J, Favus M. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. N Engl J Med. 1995;333(22):1437–43. doi: 10.1056/NEJM199511303332201. - DOI - PubMed
-
- Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348:1535–41. doi: 10.1016/S0140-6736(96)07088-2. - DOI - PubMed
-
- Meunier PJ, Delmas PD, Eastell R, McClung MR, Papapoulos S, Rizzoli R, Seeman E, Wasnich RD. Diagnosis and management of osteoporosis in postmenopausal women: clinical guidelines. International Committee for Osteoporosis Clinical Guidelines. Clin Ther. 1999;21(6):1025–44. doi: 10.1016/S0149-2918(99)80022-8. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources