Transcription and translation of human F11R gene are required for an initial step of atherogenesis induced by inflammatory cytokines
- PMID: 21703019
- PMCID: PMC3142510
- DOI: 10.1186/1479-5876-9-98
Transcription and translation of human F11R gene are required for an initial step of atherogenesis induced by inflammatory cytokines
Abstract
Background: The F11 Receptor (F11R; aka JAM-A, JAM-1) is a cell adhesion protein present constitutively on the membrane surface of circulating platelets and within tight junctions of endothelial cells (ECs). Previous reports demonstrated that exposure of ECs to pro-inflammatory cytokines causes insertion of F11R molecules into the luminal surface of ECs, ensuing with homologous interactions between F11R molecules of platelets and ECs, and a resultant adhesion of platelets to the inflamed ECs. The main new finding of the present report is that the first step in this chain of events is the de-novo transcription and translation of F11R molecules, induced in ECs by exposure to inflammatory cytokines.
Methods: The experimental approach utilized isolated, washed human platelet suspensions and cultured human venous endothelial cells (HUVEC) and human arterial endothelial cells (HAEC) exposed to the proinflammatory cytokines TNF-alpha and/or IFN-gamma, for examination of the ability of human platelets to adhere to the inflamed ECs thru the F11R. Our strategy was based on testing the effects of the following inhibitors on this activity: general mRNA synthesis inhibitors, inhibitors of the NF-kappaB and JAK/STAT pathways, and small interfering F11R-mRNA (siRNAs) to specifically silence the F11R gene.
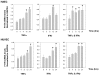
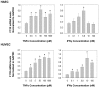
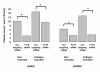
Results: Treatment of inflamed ECs with the inhibitors actinomycin, parthenolide or with AG-480 resulted in complete blockade of F11R- mRNA expression, indicating the involvement of NF-kappaB and JAK/STAT pathways in this induction. Transfection of ECs with F11R siRNAs caused complete inhibition of the cytokine-induced upregulation of F11R mRNA and inhibition of detection of the newly- translated F11R molecules in cytokine-inflamed ECs. The functional consequence of the inhibition of F11R transcription and translation was the significant blockade of the adhesion of human platelets to inflamed ECs.
Conclusion: These results prove that de novo synthesis of F11R in ECs is required for the adhesion of platelets to inflamed ECs. Because platelet adhesion to an inflamed endothelium is crucial for plaque formation in non-denuded blood vessels, we conclude that the de-novo translation of F11R is a crucial early step in the initiation of atherogenesis, leading to atherosclerosis, heart attacks and stroke.
Figures
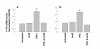



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

