Hepatocyte γ-catenin compensates for conditionally deleted β-catenin at adherens junctions
- PMID: 21703193
- PMCID: PMC3221911
- DOI: 10.1016/j.jhep.2011.03.014
Hepatocyte γ-catenin compensates for conditionally deleted β-catenin at adherens junctions
Abstract
Background & aims: Wnt/β-catenin signaling is important in liver physiology. Moreover, β-catenin is also pivotal in adherens junctions (AJ). Here, we investigate hepatocyte-specific β-catenin conditional null mice (KO) for any alterations in AJ and related tight junctions (TJ).
Methods: Using gene array, PCR, Western blot, immunohistochemistry, immunofluorescence, and co-immunoprecipitation, we compare and contrast the composition of AJ and TJ in KO and littermate wild-type (WT) control livers.
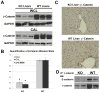
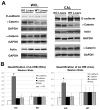
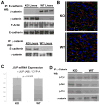
Results: We show association of E-cadherin with β-catenin in epithelial cells of WT livers, which is lost in the KOs. While total levels of α-catenin, E-cadherin, and F-actin were modestly decreased, KO livers show increased γ-catenin/plakoglobin. By co-immunoprecipitation, E-cadherin/β-catenin/F-actin association was observed in WT livers, while the association of E-cadherin/γ-catenin/F-actin was evident in KO livers. γ-Catenin was localized at the hepatocyte membrane at baseline in the KO liver. While γ-catenin gene expression remained unaltered, an increase in serine- and threonine-phosphorylated, but not tyrosine-phosphorylated γ-catenin was observed in KO livers. A continued presence of γ-catenin at the hepatocyte membrane, without any nuclear localization, was observed in liver regeneration after partial hepatectomy at 40 and 72 h, in both KO and WT. Analysis of TJ revealed lack of claudin-2 and increased levels of JAM-A and claudin-1 in KO livers.
Conclusions: β-Catenin adequately maintains AJ in the absence of β-catenin in hepatocytes; however, it lacks nuclear localization. Moreover, β-catenin/claudin-2 may be an important mechanism of crosstalk between the AJ and TJ.
Copyright © 2011 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures

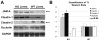
References
-
- Aberle H, Schwartz H, Kemler R. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem. 1996;61(4):514–523. - PubMed
-
- Benhamouche S, Decaens T, Godard C, Chambrey R, Rickman DS, Moinard C, et al. Apc tumor suppressor gene is the “zonation-keeper” of mouse liver. Dev Cell. 2006;10(6):759–770. - PubMed
-
- Bierkamp C, Schwarz H, Huber O, Kemler R. Desmosomal localization of beta-catenin in the skin of plakoglobin null-mutant mice. Development. 1999;126(2):371–381. - PubMed
-
- Birchmeier W, Weidner KM, Behrens J. Molecular mechanisms leading to loss of differentiation and gain of invasiveness in epithelial cells. J Cell Sci Suppl. 1993;17:159–164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

