Cytosolic phospholipase A(2)α protects against Fas- but not LPS-induced liver injury
- PMID: 21703211
- PMCID: PMC3221788
- DOI: 10.1016/j.jhep.2011.03.017
Cytosolic phospholipase A(2)α protects against Fas- but not LPS-induced liver injury
Abstract
Background & aims: Cytosolic phospholipase A(2)α (cPLA(2)α) is a rate-limiting key enzyme controlling the release of arachidonic acid (AA) substrate for the synthesis of prostaglandins and leukotrienes. This study was designed to explore the role of hepatocyte cPLA(2)α in Fas-mediated liver injury, in vivo.
Methods: Transgenic mice with targeted expression of cPLA(2)α under control of the albumin-promoter enhancer and wild-type mice were injected intraperitoneally with anti-Fas antibody Jo2 or lipopolysaccharide plus d-galactosamine and monitored for liver injury and survival at various time points.
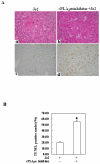
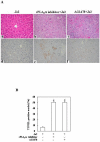
Results: The cPLA(2)α Tg mice resist Fas-induced liver failure, as reflected by the lower serum transaminase levels, fewer apoptotic hepatocytes, reduced caspase activation, and reduced PARP cleavage when compared to the matched wild type mice. Inhibition of cPLA(2)α by its pharmacological inhibitor, pyrrolidine, enhanced Jo2-induced liver injury in both cPLA(2)α Tg and wild type mice. Hepatic overexpression of cPLA(2)α increases the expression of EGFR in the liver and the EGFR inhibitor, AG1478, exacerbated Jo2-mediated liver injury. The cPLA(2)α transgenic mice develop more prominent liver tissue damage than wild-type mice after LPS/d-galactosamine injection.
Conclusions: Hepatocyte cPLA(2)α protects against Fas-induced liver injury and this effect is mediated at least in part through the upregulation of EGFR.
Copyright © 2011 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Figures









References
-
- Adachi T, Nakashima S, Saji S, Nakamura T, Nozawa Y. Roles of prostaglandin production and mitogen-activated protein kinase activation in hepatocyte growth factor-mediated rat hepatocyte proliferation. Hepatology. 1995;21(6):1668–1674. - PubMed
-
- Andreis PG, Whitfield JF, Armato U. Stimulation of DNA synthesis and mitosis of hepatocytes in primary cultures of neonatal rat liver by arachidonic acid and prostaglandins. Exp Cell Res. 1981 Aug;134(2):265–272. - PubMed
-
- Atsumi G, Tajima M, Hadano A, Nakatani Y, Murakami M, Kudo I. Fas-induced arachidonic acid release is mediated by Ca2+-independent phospholipase A2 but not cytosolic phospholipase A2, which undergoes proteolytic inactivation. J Biol Chem. 1998;273(22):13870–13877. - PubMed
-
- Bell A, Chen Q, DeFrances MC, Michalopoulos GK, Zarnegar R. The five amino acid-deleted isoform of hepatocyte growth factor promotes carcinogenesis in transgenic mice. Oncogene. 1999;18(4):887–895. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

