Insulin resistance impairs nigrostriatal dopamine function
- PMID: 21703262
- PMCID: PMC3169014
- DOI: 10.1016/j.expneurol.2011.06.005
Insulin resistance impairs nigrostriatal dopamine function
Abstract
Clinical studies have indicated a link between Parkinson's disease (PD) and Type 2 Diabetes. Although preclinical studies have examined the effect of high-fat feeding on dopamine function in brain reward pathways, the effect of diet on neurotransmission in the nigrostriatal pathway, which is affected in PD and parkinsonism, is less clear. We hypothesized that a high-fat diet, which models early-stage Type 2 Diabetes, would disrupt nigrostriatal dopamine function in young adult Fischer 344 rats. Rats were fed a high fat diet (60% calories from fat) or a normal chow diet for 12 weeks. High fat-fed animals were insulin resistant compared to chow-fed controls. Potassium-evoked dopamine release and dopamine clearance were measured in the striatum using in vivo electrochemistry. Dopamine release was attenuated and dopamine clearance was diminished in the high-fat diet group compared to chow-fed rats. Magnetic resonance imaging indicated increased iron deposition in the substantia nigra of the high fat group. This finding was supported by alterations in the expression of several proteins involved in iron metabolism in the substantia nigra in this group compared to chow-fed animals. The diet-induced systemic and basal ganglia-specific changes may play a role in the observed impairment of nigrostriatal dopamine function.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
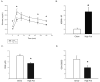


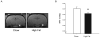

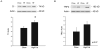

References
-
- Abbott RD, Ross GW, White LR, Nelson JS, Masaki KH, Tanner CM, Curb JD, Blanchette PL, Popper JS, Petrovitch H. Midlife adiposity and the future risk of Parkinson's disease. Neurology. 2002;59:1051–1057. - PubMed
-
- Anderson C, Checkoway H, Franklin GM, Beresford S, Smith-Weller T, Swanson PD. Dietary factors in Parkinson's disease: the role of food groups and specific foods. Mov Disord. 1999;14:21–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

