Plexiform lesions in pulmonary arterial hypertension composition, architecture, and microenvironment
- PMID: 21703400
- PMCID: PMC3123793
- DOI: 10.1016/j.ajpath.2011.03.040
Plexiform lesions in pulmonary arterial hypertension composition, architecture, and microenvironment
Abstract
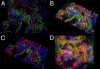
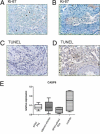
Pulmonary arterial hypertension (PAH) is a debilitating disease with a high mortality rate. A hallmark of PAH is plexiform lesions (PLs), complex vascular formations originating from remodeled pulmonary arteries. The development and significance of these lesions have been debated and are not yet fully understood. Some features of PLs resemble neoplastic disorders, and there is a striking resemblance to glomeruloid-like lesions (GLLs) in glioblastomas. To further elucidate PLs, we used in situ methods, such as (fluorescent) IHC staining, three-dimensional reconstruction, and laser microdissection, followed by mRNA expression analysis. We generated compartment-specific expression patterns in the lungs of 25 patients (11 with PAH associated with systemic shunts, 6 with idiopathic PAH, and 8 controls) and GLLs from 5 glioblastomas. PLs consisted of vascular channels lined by a continuously proliferating endothelium and backed by a uniform myogenic interstitium. They also showed up-regulation of remodeling-associated genes, such as HIF1a, TGF-β1, VEGF-α, VEGFR-1/-2, Ang-1, Tie-2, and THBS1, but also of cKIT and sprouting-associated markers, such as NOTCH and matrix metalloproteinases. The cellular composition and signaling seen in GLLs in neural neoplasms differed significantly from those in PLs. In conclusion, PLs show a distinct cellular composition and microenvironment, which contribute to the plexiform phenotype and set them apart from other processes of vascular remodeling in patients with PAH. Neoplastic models of angiogenesis seem to be of limited use in further study of plexiform vasculopathy.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Simonneau G., Galie N., Rubin L.J., Langleben D., Seeger W., Domenighetti G., Gibbs S., Lebrec D., Speich R., Beghetti M., Rich S., Fishman A. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43:5S–12S. - PubMed
-
- Pietra G.G., Edwards W.D., Kay J.M., Rich S., Kernis J., Schloo B., Ayres S.M., Bergofsky E.H., Brundage B.H., Detre K.M. Histopathology of primary pulmonary hypertension: a qualitative and quantitative study of pulmonary blood vessels from 58 patients in the National Heart, Lung, and Blood Institute, Primary Pulmonary Hypertension Registry. Circulation. 1989;80:1198–1206. - PubMed
-
- Stevens T. Molecular and cellular determinants of lung endothelial cell heterogeneity. Chest. 2005;128:558S–564S. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

