Migration of fibrocytes in fibrogenic liver injury
- PMID: 21703401
- PMCID: PMC3123781
- DOI: 10.1016/j.ajpath.2011.03.049
Migration of fibrocytes in fibrogenic liver injury
Abstract
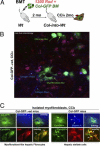
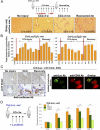
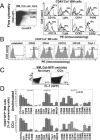
CD45(+) and collagen I-positive (Col(+)) fibrocytes are implicated in fibrogenesis in skin, lungs, and kidneys. Fibrocyte migration in response to liver injury was investigated using bone marrow (BM) from chimeric mice expressing luciferase (Col-Luc→wt) or green fluorescent protein (Col-GFP→wt) under control of the α1(I) collagen promoter and enhancer, respectively. Monitored by luciferase expression, recruitment of fibrocytes was detected in CCl(4)-damaged liver and in spleen. Migration of CD45(+)Col(+) fibrocytes was regulated by chemokine receptors CCR2 and CCR1, as demonstrated, respectively, by 50% and 25% inhibition of fibrocyte migration in Col-Luc(CCR2-/-)→wt and Col-Luc(CCR1-/-)→wt mice. In addition to CCR2 and CCR1, egress of BM CD45(+)Col(+) cells was regulated by transforming growth factor-β1 (TGF-β1) and liposaccharide in vitro and in vivo, which suggests that release of TGF-β1 and increased intestinal permeability have important roles in fibrocyte trafficking. In the injured liver, fibrocytes gave rise to (myo)fibroblasts. In addition, a BM population of CD45(+)Col(+) cells capable of differentiation into fibrocytes in culture was identified. Egress of CD45(+)Col(+) cells from BM was detected in the absence of injury or stress in aged mice but not in young mice. Development of liver fibrosis was also increased in aged mice and correlated with high numbers of liver fibrocytes. In conclusion, in response to liver injury, fibrocytes migrate from BM to the liver. Their migration is regulated by CCR2 and CCR1 but is compromised with age.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Seki E., De Minicis S., Osterreicher C.H., Kluwe J., Osawa Y., Brenner D.A., Schwabe R.F. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. - PubMed
-
- Kisseleva T., Uchinami H., Feirt N., Quintana-Bustamante O., Segovia J.C., Schwabe R.F., Brenner D.A. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol. 2006;45:429–438. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

