IL-17-induced pulmonary pathogenesis during respiratory viral infection and exacerbation of allergic disease
- PMID: 21703407
- PMCID: PMC3123803
- DOI: 10.1016/j.ajpath.2011.03.003
IL-17-induced pulmonary pathogenesis during respiratory viral infection and exacerbation of allergic disease
Abstract
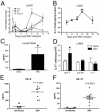
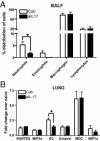
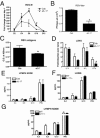
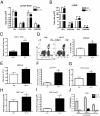
Severe respiratory syncytial virus (RSV) infections are characterized by airway epithelial cell damage, mucus hypersecretion, and Th2 cytokine production. Less is known about the role of IL-17. We observed increased IL-6 and IL-17 levels in tracheal aspirate samples from severely ill infants with RSV infection. In a mouse model of RSV infection, time-dependent increases in pulmonary IL-6, IL-23, and IL-17 expression were observed. Neutralization of IL-17 during infection and observations from IL-17(-/-) knockout mice resulted in significant inhibition of mucus production during RSV infection. RSV-infected animals treated with anti-IL-17 had reduced inflammation and decreased viral load, compared with control antibody-treated mice. Blocking IL-17 during infection resulted in significantly increased RSV-specific CD8 T cells. Factors associated with CD8 cytotoxic T lymphocytes, T-bet, IFN-γ, eomesodermin, and granzyme B were significantly up-regulated after IL-17 blockade. Additionally, in vitro analyses suggest that IL-17 directly inhibits T-bet, eomesodermin, and IFN-γ in CD8 T cells. The role of IL-17 was also investigated in RSV-induced exacerbation of allergic airway responses, in which neutralization of IL-17 led to a significant decrease in the exacerbated disease, including reduced mucus production and Th2 cytokines, with decreased viral proteins. Taken together, our data demonstrate that IL-17 plays a pathogenic role during RSV infections.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Openshaw P.J., Dean G.S., Culley F.J. Links between respiratory syncytial virus bronchiolitis and childhood asthma: clinical and research approaches. Pediatr Infect Dis J. 2003;22(2 Suppl):S58–S64. discussion S64–S65. - PubMed
-
- Black C.P. Systematic review of the biology and medical management of respiratory syncytial virus infection. Respir Care. 2003;48:209–231. discussion 231–233. - PubMed
-
- Stensballe L.G., Devasundaram J.K., Simoes E.A. Respiratory syncytial virus epidemics: the ups and downs of a seasonal virus. Pediatr Infect Dis J. 2003;22(2 Suppl):S21–S32. - PubMed
-
- Jafri H.S., Chavez-Bueno S., Mejias A., Gomez A.M., Rios A.M., Nassi S.S., Yusuf M., Kapur P., Hardy R.D., Hatfield J., Rogers B.B., Krisher K., Ramilo O. Respiratory syncytial virus induces pneumonia, cytokine response, airway obstruction, and chronic inflammatory infiltrates associated with long-term airway hyperresponsiveness in mice. J Infect Dis. 2004;189:1856–1865. - PubMed
-
- Schwarze J., Cieslewicz G., Hamelmann E., Joetham A., Shultz L.D., Lamers M.C., Gelfand E.W. IL-5 and eosinophils are essential for the development of airway hyperresponsiveness following acute respiratory syncytial virus infection. J Immunol. 1999;162:2997–3004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

