Cavia porcellus as a model for experimental infection by Trypanosoma cruzi
- PMID: 21703410
- PMCID: PMC3123796
- DOI: 10.1016/j.ajpath.2011.03.043
Cavia porcellus as a model for experimental infection by Trypanosoma cruzi
Abstract
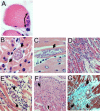
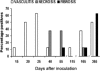
The guinea pig (Cavia porcellus) is a natural reservoir for Trypanosoma cruzi but has seldom been used as an experimental infection model. We developed a guinea pig infection model for acute and chronic Chagas disease. Seventy-two guinea pigs were inoculated intradermally with 10(4) trypomastigotes of T. cruzi strain Y (experimental group); 18 guinea pigs were used as control group. Eight animals from the experimental group and two from the control group were sacrificed 5, 15, 20, 25, 40, 55, 115, 165, and 365 days after inoculation. During the acute phase (15 to 55 days), we observed parasitemia (with a peak on day 20) and positive IgM and IgG Western blots with anti-shed acute-phase antigen bands. The cardiac tissue showed vasculitis, necrosis (on days 40 to 55), moderate to severe inflammation, and abundant amastigote nests. Smaller numbers of amastigote nests were also present in kidney, brain, and other organs. In the early chronic phase (115 to 165 days), parasitemia disappeared and anti-T. cruzi IgG antibodies were still detectable. In cardiac tissue, the number of amastigote nests and the grade of inflammation decreased. In the chronic phase (365 days), the cardiac tissue showed vasculitis and fibrosis; detectable parasite DNA was associated with higher grades of inflammation. The experimental T. cruzi infection model in guinea pigs shows kinetics and pathologic changes similar to those of the human disease.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures

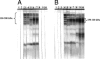


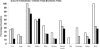
References
-
- Organización Panamericana de la Salud . Organización Panamericana de la Salud; Montevideo, Uruguay: 2006. Estimación cuantitativa de la enfermedad de Chagas en las Américas.
-
- Prata A. Clinical and epidemiological aspects of Chagas disease. Lancet Infect Dis. 2001;1:92–100. - PubMed
-
- Rassi A., Jr, Rassi S.G., Rassi A. Sudden death in Chagas' disease. Arq Bras Cardiol. 2001;76:75–96. - PubMed
-
- de Oliveira R.B., Troncon L.E., Dantas R.O., Menghelli U.G. Gastrointestinal manifestations of Chagas' disease. Am J Gastroenterol. 1998;93:884–889. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

