Dual functions of prostaglandin D2 in murine contact hypersensitivity via DP and CRTH2
- PMID: 21703412
- PMCID: PMC3123801
- DOI: 10.1016/j.ajpath.2011.03.047
Dual functions of prostaglandin D2 in murine contact hypersensitivity via DP and CRTH2
Erratum in
- Am J Pathol. 2012 Jan;180(1):429-30
Abstract
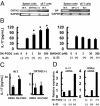
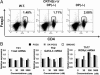
Prostaglandin D2 (PGD2) exerts its effects through two distinct receptors: the chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2) and the D prostanoid (DP) receptor. Our previous study demonstrated that CRTH2 mediates contact hypersensitivity (CHS) in mice. However, the function of DP receptor remains to be fully established. In this study, we examine the pathophysiological roles of PGD2 using DP-deficient (DP(-/-)) and CRTH2/DP-deficient (CRTH2(-/-)/DP(-/-)) mice to elucidate receptor-mediated PGD2 action in CHS. We observed profound exacerbation of CHS in DP(-/-) mice. CRTH2(-/-)/DP(-/-) mice showed similar exacerbation, but to a lesser extent. These symptoms were accompanied by increased production of interferon-γ and IL-17. The increase in IL-17 producing γδ T cells was marked and presumably contributed to the enhanced CHS. DP deficiency promoted the in vivo migration of dendritic cells to regional lymph nodes. A DP agonist added to DCs in vitro was able to inhibit production of IL-12 and IL-1β. Interestingly, production of IL-10 in dendritic cells was elevated via the DP pathway, but it was lowered by the CRTH2 pathway. Collectively, PGD2 signals through CRTH2 to mediate CHS inflammation, and conversely, DP signals to exert inhibitory effects on CHS. Thus, we report opposing functions for PGD2 that depend on receptor usage in allergic reactions.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
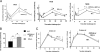




Similar articles
-
Distinct roles of prostaglandin D2 receptors in chronic skin inflammation.Mol Immunol. 2011 Oct;49(1-2):304-10. doi: 10.1016/j.molimm.2011.08.023. Epub 2011 Sep 23. Mol Immunol. 2011. PMID: 21943706
-
Differential modulation of human basophil functions through prostaglandin D2 receptors DP and chemoattractant receptor-homologous molecule expressed on Th2 cells/DP2.Clin Exp Allergy. 2004 Aug;34(8):1283-90. doi: 10.1111/j.1365-2222.2004.02027.x. Clin Exp Allergy. 2004. PMID: 15298571
-
Lipopolysaccharide induces proinflammatory cytokines and chemokines in experimental otitis media through the prostaglandin D2 receptor (DP)-dependent pathway.Clin Exp Immunol. 2011 Feb;163(2):260-9. doi: 10.1111/j.1365-2249.2010.04292.x. Epub 2010 Dec 16. Clin Exp Immunol. 2011. PMID: 21166666 Free PMC article.
-
Prostaglandin D2 receptors DP and CRTH2 in the pathogenesis of asthma.Curr Mol Med. 2008 Aug;8(5):365-75. doi: 10.2174/156652408785160970. Curr Mol Med. 2008. PMID: 18691063 Review.
-
[Prostaglandin D2 in allergy: PGD2 has dual receptor systems].Nihon Yakurigaku Zasshi. 2004 Jan;123(1):15-22. doi: 10.1254/fpj.123.15. Nihon Yakurigaku Zasshi. 2004. PMID: 14695454 Review. Japanese.
Cited by
-
Indomethacin inhibits eosinophil migration to prostaglandin D2 : therapeutic potential of CRTH2 desensitization for eosinophilic pustular folliculitis.Immunology. 2013 Sep;140(1):78-86. doi: 10.1111/imm.12112. Immunology. 2013. PMID: 23582181 Free PMC article.
-
Prostaglandin D2 receptor D-type prostanoid receptor 2 mediates eosinophil trafficking into the esophagus.Dis Esophagus. 2014 Aug;27(6):601-6. doi: 10.1111/dote.12118. Epub 2013 Oct 25. Dis Esophagus. 2014. PMID: 24165271 Free PMC article.
-
The CRTH2 agonist Pyl A prevents lipopolysaccharide-induced fetal death but induces preterm labour.Immunology. 2013 Jul;139(3):352-65. doi: 10.1111/imm.12085. Immunology. 2013. PMID: 23374103 Free PMC article.
-
PGD2 and CRTH2 counteract Type 2 cytokine-elicited intestinal epithelial responses during helminth infection.J Exp Med. 2021 Sep 6;218(9):e20202178. doi: 10.1084/jem.20202178. Epub 2021 Jul 20. J Exp Med. 2021. PMID: 34283207 Free PMC article.
-
Host Lipid Mediators in Leprosy: The Hypothesized Contributions to Pathogenesis.Front Immunol. 2018 Feb 2;9:134. doi: 10.3389/fimmu.2018.00134. eCollection 2018. Front Immunol. 2018. PMID: 29472920 Free PMC article. Review.
References
-
- Nagoshi H., Uehara Y., Kanai F., Maeda S., Ogura T., Goto A., Toyo-oka T., Esumi H., Shimizu T., Omata M. Prostaglandin D2 inhibits inducible nitric oxide synthase expression in rat vascular smooth muscle cells. Circ Res. 1998;82:204–209. - PubMed
-
- Whittle B.J., Moncada S., Vane J.R. Comparison of the effects of prostacyclin (PGI2), prostaglandin E1 and D2 on platelet aggregation in different species. Prostaglandins. 1978;16:373–388. - PubMed
-
- Kanaoka Y., Urade Y. Hematopoietic prostaglandin D synthase. Prostaglandins Leukot Essent Fatty Acids. 2003;69:163–167. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

