Time-course and regional analyses of the physiopathological changes induced after cerebral injection of an amyloid β fragment in rats
- PMID: 21703413
- PMCID: PMC3123848
- DOI: 10.1016/j.ajpath.2011.03.021
Time-course and regional analyses of the physiopathological changes induced after cerebral injection of an amyloid β fragment in rats
Abstract
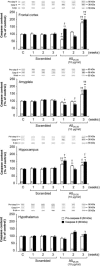
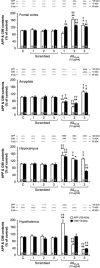
Alzheimer's disease (AD) is a neurodegenerative pathology characterized by the presence of senile plaques and neurofibrillary tangles, accompanied by synaptic and neuronal loss. The major component of senile plaques is an amyloid β protein (Aβ) formed by pathological processing of the Aβ precursor protein. We assessed the time-course and regional effects of a single intracerebroventricular injection of aggregated Aβ fragment 25-35 (Aβ(25-35)) in rats. Using a combined biochemical, behavioral, and morphological approach, we analyzed the peptide effects after 1, 2, and 3 weeks in the hippocampus, cortex, amygdala, and hypothalamus. The scrambled Aβ(25-35) peptide was used as negative control. The aggregated forms of Aβ peptides were first characterized using electron microscopy, infrared spectroscopy, and Congo Red staining. Intracerebroventricular injection of Aβ(25-35) decreased body weight, induced short- and long-term memory impairments, increased endocrine stress, cerebral oxidative and cellular stress, neuroinflammation, and neuroprotective reactions, and modified endogenous amyloid processing, with specific time-course and regional responses. Moreover, Aβ(25-35), the presence of which was shown in the different brain structures and over 3 weeks, provoked a rapid glial activation, acetylcholine homeostasis perturbation, and hippocampal morphological alterations. In conclusion, the acute intracerebroventricular Aβ(25-35) injection induced substantial central modifications in rats, highly reminiscent of the human physiopathology, that could contribute to physiological and cognitive deficits observed in AD.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures











Similar articles
-
Alzheimer's disease like pathology induced six weeks after aggregated amyloid-beta injection in rats: increased oxidative stress and impaired long-term memory with anxiety-like behavior.Neurol Res. 2016 Sep;38(9):838-50. doi: 10.1080/01616412.2016.1209337. Epub 2016 Jul 19. Neurol Res. 2016. PMID: 27431920
-
Alzheimer's disease related markers, cellular toxicity and behavioral deficits induced six weeks after oligomeric amyloid-β peptide injection in rats.PLoS One. 2013;8(1):e53117. doi: 10.1371/journal.pone.0053117. Epub 2013 Jan 2. PLoS One. 2013. PMID: 23301030 Free PMC article.
-
Probucol, a lipid-lowering drug, prevents cognitive and hippocampal synaptic impairments induced by amyloid β peptide in mice.Exp Neurol. 2012 Feb;233(2):767-75. doi: 10.1016/j.expneurol.2011.11.036. Epub 2011 Dec 8. Exp Neurol. 2012. PMID: 22173317
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
Is Alzheimer's disease a result of presynaptic failure? Synaptic dysfunctions induced by oligomeric beta-amyloid.Rev Neurosci. 2009;20(1):1-12. doi: 10.1515/revneuro.2009.20.1.1. Rev Neurosci. 2009. PMID: 19526730 Review.
Cited by
-
Na+ and K+ ion imbalances in Alzheimer's disease.Biochim Biophys Acta. 2012 Nov;1822(11):1671-81. doi: 10.1016/j.bbadis.2012.07.004. Epub 2012 Jul 20. Biochim Biophys Acta. 2012. PMID: 22820549 Free PMC article.
-
The selective GSK3 inhibitor, SAR502250, displays neuroprotective activity and attenuates behavioral impairments in models of neuropsychiatric symptoms of Alzheimer's disease in rodents.Sci Rep. 2019 Dec 2;9(1):18045. doi: 10.1038/s41598-019-54557-5. Sci Rep. 2019. PMID: 31792284 Free PMC article.
-
Protective Effects of 6-(Methylsulfinyl)hexyl Isothiocyanate on Aβ1-42-Induced Cognitive Deficit, Oxidative Stress, Inflammation, and Apoptosis in Mice.Int J Mol Sci. 2018 Jul 18;19(7):2083. doi: 10.3390/ijms19072083. Int J Mol Sci. 2018. PMID: 30021941 Free PMC article.
-
Hippocampal injections of soluble amyloid-beta oligomers alter electroencephalographic activity during wake and slow-wave sleep in rats.Alzheimers Res Ther. 2023 Oct 13;15(1):174. doi: 10.1186/s13195-023-01316-4. Alzheimers Res Ther. 2023. PMID: 37833786 Free PMC article.
-
Studies for Improving a Rat Model of Alzheimer's Disease: Icv Administration of Well-Characterized β-Amyloid 1-42 Oligomers Induce Dysfunction in Spatial Memory.Molecules. 2017 Nov 18;22(11):2007. doi: 10.3390/molecules22112007. Molecules. 2017. PMID: 29156571 Free PMC article.
References
-
- Selkoe D.J. Translating cell biology into therapeutic advances in Alzheimer's disease. Nature. 1999;399(6738 Suppl):A23–A31. - PubMed
-
- Yankner B.A. Mechanisms of neuronal degeneration in Alzheimer's disease. Neuron. 1996;16:921–932. - PubMed
-
- Selkoe D.J. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. - PubMed
-
- Pike C.J., Cummings B.J., Cotman C.W. Beta-amyloid induces neuritic dystrophy in vitro: similarities with Alzheimer pathology. Neuroreport. 1992;3:769–772. - PubMed
-
- Pike C.J., Walencewicz-Wasserman A.J., Kosmoski J., Cribbs D.H., Glabe C.G., Cotman C.W. Structure-activity analyses of beta-amyloid peptides: contributions of the beta 25–35 region to aggregation and neurotoxicity. J Neurochem. 1995;64:253–265. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

