The role of engineered tendon matrix in the stemness of tendon stem cells in vitro and the promotion of tendon-like tissue formation in vivo
- PMID: 21703682
- PMCID: PMC3148341
- DOI: 10.1016/j.biomaterials.2011.05.088
The role of engineered tendon matrix in the stemness of tendon stem cells in vitro and the promotion of tendon-like tissue formation in vivo
Abstract
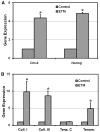
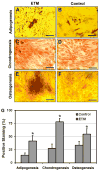
When injured, tendons tend to heal but with poor structure and compromised function. Tissue engineering is a promising approach to enhancing the quality of healing tendons. Our group and others have identified tendon stem cells (TSCs), a type of tendon-specific stem cells which may be optimal for cellular interventions seeking to restore normal structure and function to injured tendons. However, in vitro expanding of TSCs on regular plastic cell culture dishes only yields a limited number of TSCs before they lose the stemness, i.e., the self-renewal capability and multipotency. In this study, we developed a substrate material for TSCs, engineered tendon matrix (ETM) from decellularized tendon tissues. We showed that ETM in vitro was able to stimulate TSC proliferation and better preserve the stemness of TSCs than plastic culture surfaces. In vivo, implantation of ETM-TSC composite promoted tendon-like tissue formation whereas implantation of TSCs alone led to little such tissue formation. Together, the findings of this study indicate that ETM may be used to effectively expand TSCs in vitro and with TSCs, to enhance repair of injured tendons in vivo.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures





References
-
- Butler DL, Juncosa N, Dressler MR. Functional efficacy of tendon repair processes. Annu Rev Biomed Eng. 2004;6:303–29. - PubMed
-
- Butler DL, Juncosa-Melvin N, Boivin GP, Galloway MT, Shearn JT, Gooch C, et al. Functional tissue engineering for tendon repair: A multidisciplinary strategy using mesenchymal stem cells, bioscaffolds, and mechanical stimulation. J Orthop Res. 2008;26:1–9. - PubMed
-
- Chen X, Zou XH, Yin GL, Ouyang HW. Tendon tissue engineering with mesenchymal stem cells and biografts: an option for large tendon defects? Front Biosci (Schol Ed) 2009;1:23–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

