Acoustic droplet vaporization for enhancement of thermal ablation by high intensity focused ultrasound
- PMID: 21703883
- PMCID: PMC3152672
- DOI: 10.1016/j.acra.2011.04.012
Acoustic droplet vaporization for enhancement of thermal ablation by high intensity focused ultrasound
Abstract
Rationale and objectives: Acoustic droplet vaporization (ADV) shows promise for spatial control and acceleration of thermal lesion production. The investigators hypothesized that microbubbles generated by ADV could enhance high-intensity focused ultrasound (HIFU) thermal ablation by controlling and increasing local energy absorption.
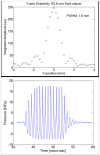
Materials and methods: Thermal lesions were produced in tissue-mimicking phantoms using focused ultrasound (1.44 MHz) with a focal intensity of 4000 W · cm(-2) in degassed water at 37°C. The average lesion volume was measured by visible change in optical opacity and by T2-weighted magnetic resonance imaging. In addition, in vivo HIFU lesions were generated in a canine liver before and after an intravenous injection of droplets with a similar acoustic setup.
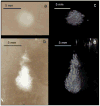
Results: Thermal lesions were sevenfold larger in phantoms containing droplets (3 × 10(5) droplets/mL) compared to phantoms without droplets. The mean lesion volume with a 2-second HIFU exposure in droplet-containing phantoms was comparable to that made by a 5-second exposure in phantoms without droplets. In the in vivo study, the average lesion volumes without and with droplets were 0.017 ± 0.006 cm(3) (n = 4; 5-second exposure) and 0.265 ± 0.005 cm(3) (n = 3; 5-second exposure), respectively, a factor of 15 difference. The shape of ADV bubbles imaged with B-mode ultrasound was very similar to the actual lesion shape as measured optically and by magnetic resonance imaging.
Conclusion: ADV bubbles may facilitate clinical HIFU ablation by reducing treatment time or requisite in situ total acoustic power and provide ultrasonic imaging feedback of the thermal therapy.
Copyright © 2011 AUR. Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Boyle P, Levin B, editors. World Cancer Report 2008. Lyon, France: IARC Press; 2008. p. 350.
-
- Yamasaki S, Hasegawa H, Makuuchi M, Takayama T, Kosuge T, Shimada K. Choice of treatments for small hepatocellular carcinoma: hepatectomy, embolization or ethanol injection. J Gastroenterol Hepatol. 1991;6(4):408–13. - PubMed
-
- Molinari M, Helton S. Hepatic resection versus radiofrequency ablation for hepatocellular carcinoma in cirrhotic individuals not candidates for liver transplantation: a Markov model decision analysis. Am J Surg. 2009;198(3):396–406. - PubMed
-
- Cancer Facts and Figures. American Cancer Society; 2004.
-
- Hill CR, ter Haar GR. Review article: high intensity focused ultrasound--potential for cancer treatment. Br J Radiol. 1995;68(816):1296–303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

